Team:Nagahama/Protocol
Contents
Protocols
Our Lab's Protocols
Medium
LB medium (100 mL liquid)
1.Measure 1g Tripton
2.Measure 0.5g Yeast Extract
3.Measure 1g Nacl
4.Add 100mL H2O
5.autoclave(121℃ 20min)
2×YT medium (100mL liquid)
1.Measure 1.6g Tripton
2.Measure 1g Yeast Extract
3.Measure 0.5g Nacl
4.Add 100mL H2O
5.autoclave(121℃ 20min)
DNA work
Agarose gel(100mL)
Method of Making 0.7% Agarose gel
1.Measure 0.7g Agarose
2.Add 100mL TAE buffer
3.Heat(till agarose melted)*We used a microwave oven.
4.Pur agarose into a gel maker
5.Set a comb
6.Wait till agarose curdles
7.Pull an comb
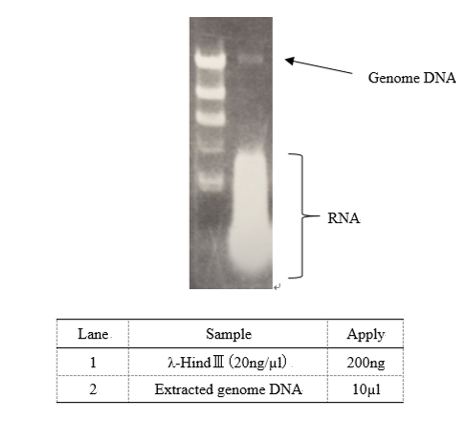
Genome DNA extraction
↓Cultivate E. coli DH5α using LB medium 2ml×2 tubes O/N
↓Centrifuge culture 1.5ml (13,000rpm 4℃ 1min)
↓Remove the culture
↓+TNE buffer 1.0ml (10mM Tris pH 8.0, 10 mM NaCl, 10 mM EDTA)
↓vortex
↓Centrifuge cell suspension (13,000rpm 4℃ 2min)
↓Remove supernatant
↓+TNE buffer including 1% Triton X-100 270µl
↓Suspend cell gently
↓+5mg/ml Lysozyme solution 30µl (0.15g Lyzozyme + 30µl sterile water)
↓Reaction 37℃ 30min
↓+ 20mg/ml Proteinase K (fin.con: 1mg/ml) TaKaRa Code:9033
↓Reaction 65℃ 2h
↓+Phenol chloroform 300µl ...(1)
↓Mix the solution gently ...(2)
↓Centrifuge (13,000rpm 4℃ 8min) ...(3)
↓Transfer only water layer to new 1.5ml tube ...(4)
↓Repeat (1)~(4) 2 times
↓+3M Sodium acetate 30µl
↓Mix gently
↓+99.5% EtOH 750µl
↓Mix gently
↓Centrifuge (13,000rpm 4℃ 10min)
↓Remove supernatant
↓+70% EtOH 500µl
↓Centrifuge (13,000rpm 4℃ 1min)
↓Remove supernatant
↓Dry up the pellet covering with aluminum foil at room temperature 30min
↓+TE buffer 50µl
↓Suspend DNA gently
Plasmids extraction
PCR
Transformation by heat shock
Proof of concept(POC)
Result here
Preventive effect of grated garlic
We prepared 20g pork and 5g garlic. We left the pork for an hour on the table and grated garlics. We spread the garlic grated on the pork (A). We left the two pork in the box at 18℃ for 2 month. Left pork (A) didn’t change the color. Right pork (B) changed Pink to Brown. We found from this result that fragrance of plants have antibacteril volatiles.
Preventive effect of grated Wasabi root
We prepared some rice cake and 10g wasabi. We left the rice cake for an hour on the table and grated wasabi. We spread the wasabi grated on the rice cake (A). We left each rice cake in the box at 18℃ for 2 month. Rice cake of the above (A) didn’t change the color. Rice cake under (B) changed white to Black. We found from this result that fragrance of plants have antibacteril volatiles.
Confirmation of anti-mold and antibocatrial activities of geraniol and farnesol
Storage of food by geraniol A:ddH2O(300 μl) B:geraniol(stock solution, 300 μl) Incubation: 35 days Temperature: room temperature Chopsticks dipped in suspension of Penicillium was put in the center of the bread.
Penicillium was cultured among the left box(A), and Penicillium wasn’t cultured among the right box(B), suggesting that Penicillium can’t be cultured in a state where geraniol is filled. This experiment indicate that geraniol might be the effect of suppressing the growth of bacteria
isoprenoid production
Because of its high hydrophobicity and low volatility, decane was chosen to extract and solubilize farnesol from the culture broth. The decane was overlaidy in the two-phase culture media, but it did not affect the cell growth, and farnesol could be well solubilized in the decane phase with negligible volatile loss. We adopt used 1 mL of decane to overlayid to the 5 mL of culture broth. Two-phase The culture of E. coli JM109 (BBa_K165025) was carried out in 2YT medium containing 1% glycerol at 29°C for 48 h. The decane phase of the two-phase culture was collected to analyze the farnesol content by GC-MS.
Analysis of ubiquinone-8 synthesized by E. coli
1. Put E. coli cells in: methanol (7: 2).
2. Sonicate cells for 30 sec. and cool down 30 sec. and collect supernatants. ( 6 times).
3. Spin down the supernatants at 12,000xg, 10min at 4 ℃, and dry up acetone and methanol.
4. Add 400μl of chloroform : methanol ( 1 : 1 ) to the dried supernatants. Add equal volume 0.7% NaCl and spin down 12,000xg 5min at 4 ℃.
5. Extract the lower layer
6. Add 100μl of chloroform : methanol (2: 1).
7. Spot the extracts on TLC plate and develop extracts using benzene : acetone (7 93).