Team:Paris Saclay/Notebook/August/11
Contents
Tuesday 11th August
Lab Work
Ligation
by Pauline
- BBa_K1707031: BBa_K1707004 + BBa_R0040
- 10,4 µL BBa_K1707004 digested by EcoRI + SpeI
- 3,3 µL BBa_R0040 digested by EcoRI + XbaI
- 2 µL Ligase
- 2 µL Buffer 10x
- 2,3 µL H2O
- BBa_K1707033: BBa_K1707006 + BBa_B0030
- 7,3 µL BBa_K1707006 digested by XbaII + PstI
- 7,4 µL BBa_B0030
- 2 µL Ligase
- 2,5 µL Buffer 10x
- 5,8 µL H2O
- BBa_K1707016: BBa_K1707004 + BBa_I13602
- 7,2 µL BBa_K1707004 digested by EcoRI + SpeI
- 2 µL BBa_B0030
- 2 µL Ligase
- 1,5 µL Buffer 10x
- 2,5 µL H2O
Incubation 4°C, 3h
Plamid Extraction
by Pauline
- BBa_K1707026 #1 and #2
- BBa_K1707032 #1 and #2
With Macherey-Nagel Extraction kit
Digestion
by Coralie
- BBa_K1707026 #1 and #2
- BBa_K1707032 #1 and #2
Mix for each reaction:
- 0,5 µL PstI
- 0,5 µL XbaI
- 1 µL Buffer FastDigest 10x
- 6 µL H2O
- 2 µL plasmid
Incubation 37°C, 2h
Electrophoresis
by Pauline
Agarose gel 1%, migration 90V
Biobricks:
- Quantification:
- BBa_K1707011 #3 (plasmid)
- BBa_K1707012 #2 (plasmid)
- Verification of digestion:
- BBa_K1707026 #1
- BBa_K1707026 #2
- BBa_K1707028 #1
- BBa_K1707028 #2
- BBa_K1707032 #1
- BBa_K1707032 #2
Wells 1-2: Quantification, Wells 4-9: Verification by digestion with XbaI and PstI; from left to right: 1. K1707011#3 plasmid, 2. K1707012#2, 3. DNA Ladder, 4. BBa_K1707026#1, 5. BBa_K1707026#2, 6. BBa_K1707028#1, 7. BBa_K1707028#2, 8. BBa_K1707032#1, 9. K1707032#1, 10. Empty
We can conclude:- Quantification:
- BBa_K1707011 #3 (plasmid): 13 µg/µL
- BBa_K1707012 #2 (plasmid): 20 µg/µL
- Verification of digestion:
- BBa_K1707026 #1: OK but not totally digested
- BBa_K1707026 #2: OK but not totally digested
- BBa_K1707028 #1: OK
- BBa_K1707028 #2: OK
- BBa_K1707032 #1: OK but not totally digested
- BBa_K1707032 #2: OK but not totally digested
Digestion
by Coralie
Biobricks:
- BBa_I13602 x2
- Mix for each reaction:
- 1 µL EcoRI
- 1 µL XbaI
- 2 µL Buffer FastDigest 10x
- 6 µL H2O
- 10 µL plasmid
- Incubation 37°C, 2h
- Mix for each reaction:
- BBa_K1707030 and BBa_K1707020
- Mix for each reaction:
- 2 µL Tango Buffer
- 1 µL SpeI
- 7 µL H2O
- 10 µL Plasmid
- Incubation 2h, 37°C
- After incubation: we add in each tube:
- 3 µL Tango Buffer
- 1 µL EcoRI
- 1 µL H2O
- Incubation 1h30, 37°C
- Mix for each reaction:
Purification on gel
by Pauline
Biobricks:
- BBa_K1707020
- BBa_K1707030
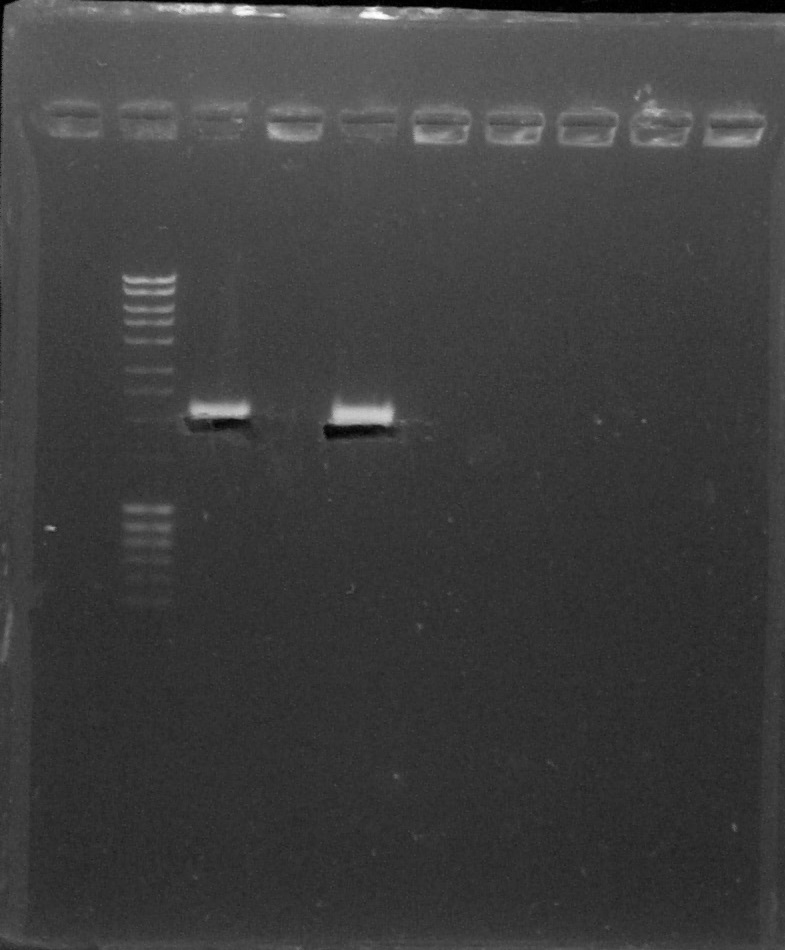
Wells 1-2: Quantification, Wells 4-9: Verification by digestion with XbaI and PstI; from left to right: 1. Empty, 2. DNA Ladder, 3. BBa_K1707020, 4. Empty, 5. BBa_K1707030, 6. Empty, 7. Empty, 8. Empty, 9. Empty, 10. Empty
We can conclude that biobricks are OKTransformation
by Pauline
Biobricks:
- BBa_K1707016
- BBa_K1707031
- BBa_K1707033
As usual
Low cost experiment
by Coralie
We make 8 plates:
- In each one:
- 0,27g Stock Cube
- 0,600g Agar Agar
- 0,135g Baker's yeast
- 45mL mineral water
We make 3 LB plates for control
We use the strain created for the Interlab Study (BBa_J23101 + GFP) to make stries on each plate. We put 3 plates in each condition (2 home-made + 1 LB plate)
- 37°C
- In a yogurt maker
- On the table
Incubation overnight
Member present:
- Instructors: Claire
- Students: Coralie and Pauline