Team:Paris Saclay/Notebook/July/16
Contents
Thursday 16th July
Lab Work
Plasmid extraction
by Johan
- BBa_S03518
- BBa_B0015
Cultures from the 07/15/2015 With the Nucleospin Kit from Macherey Nagel
Digestion
by Coralie
- BBa_S03518
- BBa_B0015
- BBa_I13602
In each tube:
- Plasmid: 10µL
- Enzyme: 1µL of each enzyme
- Buffer FastDigest (10x): 2µL
- H2O: 6µL
Enzymes choice:
- BBa_S03518 #1: XbaI + PstI
- BBa_S03518 #2: SpeI + EcoRI
- BBa_B0015 #1: PstI + SpeI
- BBa_B0015 #2: XbaI + EcoRI
- BBa_I13602 (x2): XbaI + Pst I
Incubation 1h30, 37°C
PCR
by Coralie
- BBa_R0051
We use the rehydrated plasmid from the iGEM plate 2014
Reaction mix for 3 tubes:
- GC Buffer (5x): 30µL
- dNTP (10mM): 3µL
- Forward Primer (1/10): 7,5µL
- Reverse Primer (1/10): 7,5µL
- Template DNA R0051 (2014): 5µL
- DNA Pol Phusion: 1,5µL
- H2O: 97,5µL
We use 50µL of that mix in each tube
Cycle: Initiation: 98°C - 30seconds Cycle (34 repeats): 98°C - 10seconds / 65°C - 30seconds / 72°C - 20seconds Term.: 72°C - 5min Keep it at 4°C
New culture
by Seong Koo
Observation of our plates: a lot of colony in each one. New liquid culture of:
- BBa_K1399005
- BBa_K1399019
- BBa_K1399023
- Ligation product: BBa_J23101 + BBa_K115017
2x 5ml LB + 10μl Chloramphenicol + 1 bacterial colony. We incubate cultures at 37°C, ON.
Plasmid extraction
by Pauline
- BBa_R0051
- BBa_B0030
Cultures from the 07/15/2015 With the Nucleospin Kit from Macherey Nagel
Digestion
by Pauline
- BBa_R0051 (07/15/2015)
- BBa_R0051 (07/16/2015)
- BBa_B0030
- BBa_J23101 + BBa_I13504
- BBa_J23106 + BBa_I13504
- BBa_J23117 + BBa_I13504
Reaction mix:
- Plasmid: 2µL
- EcoRI: 0,5µL
- PstI: 0,5µL
- Buffer FastDigest (10x): 2µL
- H2O: 15µL
Electrophoresis
by Pauline
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET Migration 0,06A 80V
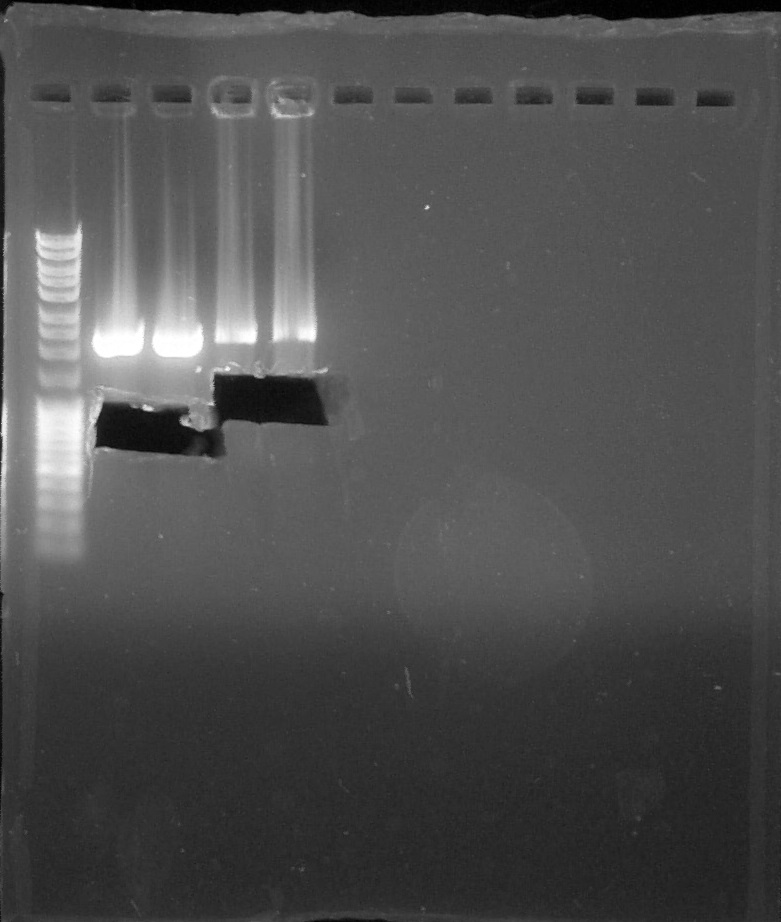
Digestion verification, from left to right: 1. DNA Ladder, 2. BBa_R0051 (15/07), 3. BBa_R0051 (16/07), 4. BBa_B0030#1, 5. BBa_J23101+I13504#1, 6. BBa_J23106+I13504#1, 7. BBa_J23117+I13504#1, 8. Empty, 9. Empty, 10. Empty
Purification of BioBricks by electrophoresis
by Coralie
- BBa_S03518
- BBa_I13602
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET Migration 0,06A 80V
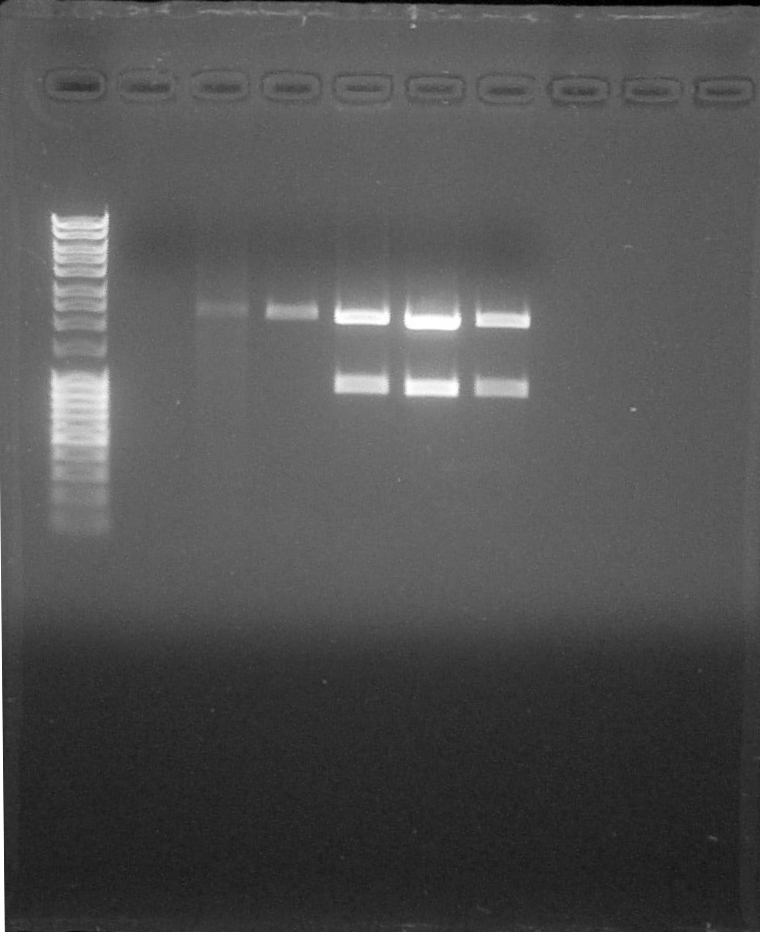
Purificatin verification, from left to right: 1. DNA Ladder, 2. BBa_S03518#1, 3. BBa_S03518#2, 4. BBa_I13602#1, 5. BBa_I13602#2, 6. Empty, 7. Empty, 8. Empty, 9. Empty, 10. Empty, 11. Empty, 12. Empty
We cut interested bands with a scalpel.DNA Extraction and purification
by Coralie
- BBa_S03518 #1 and #2
- BBa_B0015 #1 and #2
- BBa_I13602 (x2)
- BBa_R0051 (from PCR)
We use the PCR Clean Up / Gel Extraction Kit from Macherey-Nagel We elute DNA in 30 µL.
Quantification on Agarose Gel
by Johan
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET We load 2µL of previous purified DNA with 2 µL of DNA Loading (6x) and 8 µL of H2O Migration 0,06A 80V
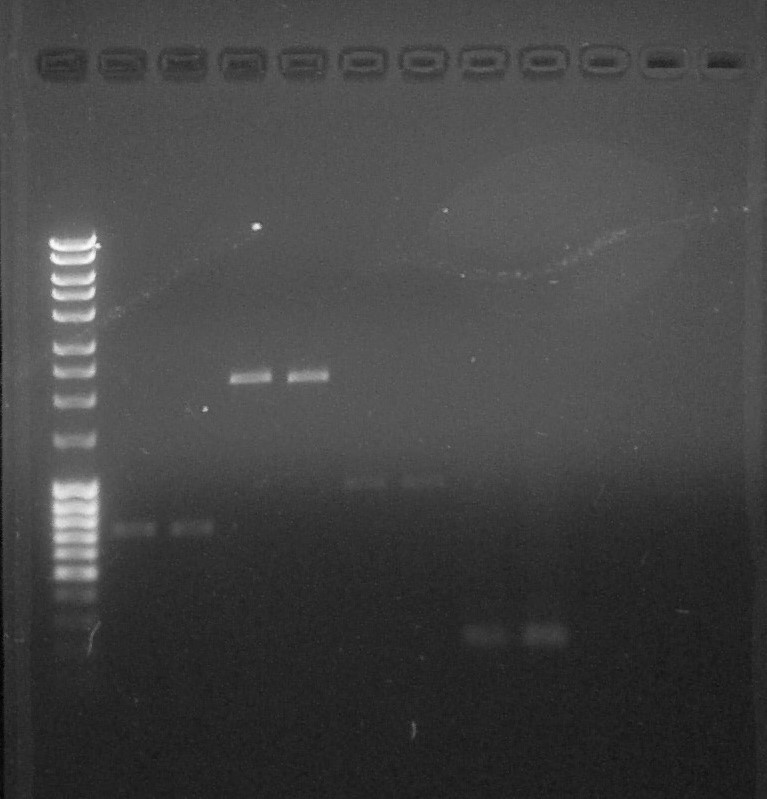
Quantification, from left to right: 1. DNA Ladder, 2. BBa_S03518#1, 3. BBa_S03518#2, 4. BBa_B0015#1, 5. BBa_B0015#2, 6. BBa_I13602#1, 7. BBa_I13602#2, 8. BBa_R0051#1, 9. BBa_BBa_R0051#2, 10. Empty, 11. Empty, 12. Empty
We can observe that the PCR of R0051 was effective. We can quantify purified DNA:- BBa_S03518 #1: 15 ng/µL
- BBa_S03518 #2: 15ng/µL
- BBa_B0015 #1: 10 ng/µL
- BBa_B0015 #2: 10 ng/µL
- BBa_I13602 (x2): 5ng/µL
- BBa_R0051: 10 ng/µL
Members present:
- Instructors and advisors: Alice.
- Students: Johan, Seong Koo, Coralie, Pauline