Team:Paris Saclay/Notebook/July/29
Contents
Wednesday 29th July
Lab Work
Plasmid extraction
by Seong-Koo
Biobricks:
- BBa_K1707008 #1 and #2
- BBa_K1707010 #1 and #2
- BBa_R0051
With the Macherey-Nagel Extraction kit
Digestion
by Pauline
- BBa_K1707008 #1
- 2µL Buffer FastDigest 10x
- 1µL SpeI
- 1µL PstI
- 10µL Plasmid
- 6µL H2O
- BBa_K1707010 #1
- 2µL Buffer FastDigest 10x
- 1µL XbaI
- 1µL PstI
- 10µL Plasmid
- 6µL H2O
- BBa_R0051
- 1µL Buffer FastDigest 10x
- 1µL SpeI
- 1µL PstI
- 2µL Plasmid
- 5µL H2O
Incubation 1h30, 37°C
Purification gel
by Pauline
Biobricks:
- BBa_K1707010 #1
Purification on agarose gel 1%, migration 100V
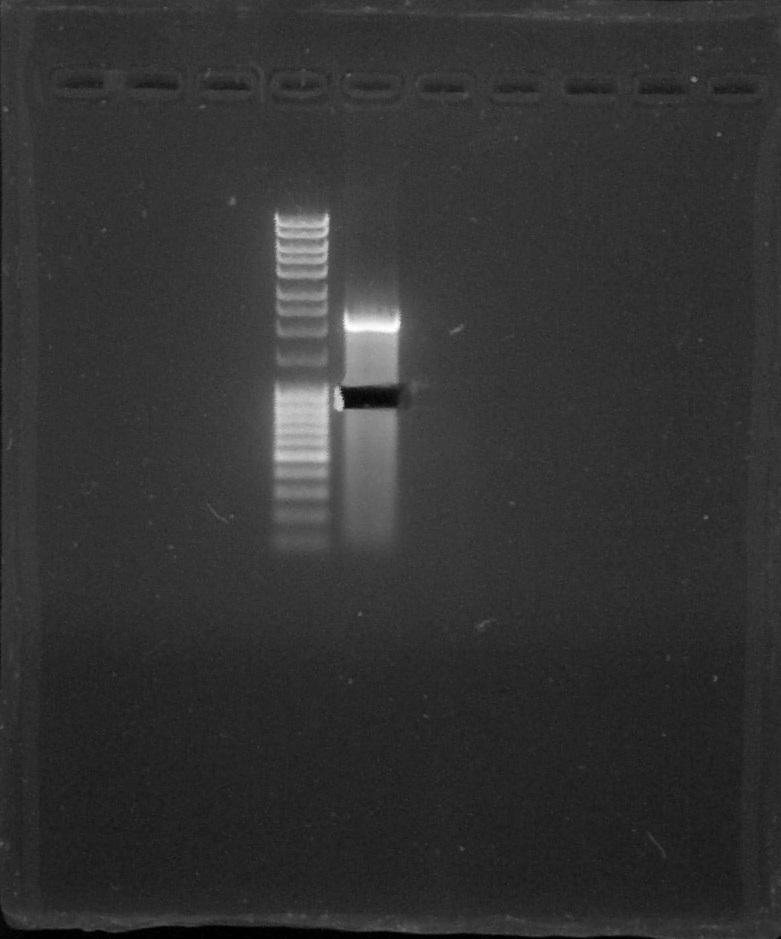
Verification of gel purification, from left to right: 1. Empty, 2. Empty, 3. Empty, 4. DNA Ladder, 5. BBa_K1707010#1, 6. Empty, 7. Empty, 8. Empty, 9. Empty, 10. Empty
Cut with a scalpel
Purification
by Pauline
Biobricks:
- BBa_K1707008 #1
- BBa_K1707010 #1
- BBa_R0051
Purification with the PCR Clean up kit from Macherey-Nagel
Digestion for verification
by Audrey
Biobricks:
- BBa_K1707005 #1 and #2
Mix:
- 2µL plasmid
- 0,5µL NotI
- 1µL Buffer FastDigest 10x
- 6,5µL H2O
Incubation 1h30, 37°C
Quantification
by Pauline
Biobricks:
- BBa_K1707003 #5
- BBa_K1707008 #1
- BBa_K1707010 #1
- BBa_R0051
Agarose gel, 1%, migration 110V
Wells 1-5: Quantification, Wells 7-8: Verification by digestion with NotI; from left to right: 1. BBa_K1707003#5, 2. BBa_K1707008#1, 3. BBa_K1707010#1, 4. BBa_R0051, 5. R0051 plasmid, 6. DNA Ladder, 7. BBa_K1707005#1, 8. BBa_K1707005#2, 9. Empty, 10. Empty, 11. Empty, 12. Empty
We can conclude that K1707005 isn't digested
We can conclude the quantification:
- BBa_K1707003 #5: 10ng/µL
- BBa_K1707008 #1: not OK
- BBa_K1707010 #1: 10 ng/µL
- BBa_R0051: 8ng/µL
Ligation
by Audrey
- BBa_K1707012: BBa_B0030 + BBa_K1707007
- 6 µL BBa_B0030
- 13 µL BBa_K1707007
- 2,5µL Buffer Ligase 10x
- 1 µL Ligase
- 2,5 µL H2O
- BBa_K1707019: BBa_K1707000 + BBa_K1707006
- 2,5µL BBa_K1707000
- 13 µL BBa_K1707006
- 2 µL Buffer Ligase 10x
- 1 µL Ligase
- 1,5 µL H2O
- BBa_K1707013: BBa_K1707000 + BBa_K1707007
- 1 µL BBa_K1707000
- 6 µL BBa_K1707007
- 1 µL Buffer Ligase 10x
- 1 µL Ligase
- 1 µL H2O
- BBa_K1707009: BBa_S03518 + BBa_R0051
- 5,5µL BBa_S03518
- 6,5 µL BBa_R0051
- 1,5µL Buffer Ligase 10x
- 1 µL Ligase
- 0,5 µL H2O
- BBa_K1707004: BBa_K1707003 + BBa_R0051
- 10 µL BBa_K1707003
- 6,5 µL BBa_K1707007
- 2 µL Buffer Ligase 10x
- 1 µL Ligase
- 0,5 µL H2O
Incubation over night, 16°C
Transformation
by Coralie
Biobricks:
- BBa_K1707012
- BBa_K1707019
- BBa_K1707013
As usual
Rehydratation and transformation
by Johan
Biobricks:
- BBa_E0022
- BBa_E0422
New Culture
by Pauline
Biobricks:
- BBa_K1707008 #3, #4, #5 and #6
Soil experiment
by Johan and Coralie
Soil
Observation of J0 plates: there are a lot of colony in each. Contamination is alright. Controls (+) and (-) are Ok too. We take 1g of each pot of soil and we dilute it in 5mL sterile H2O. After shaking and decant, we put 100µL of supernatant on the right plate. Incubation ON 37°C
Water
We can't see anything on water plates. We try another type, like yesterday but without dilution and dilution 10-1. We 100µL of different dilution on each type of plate:
- LB without antibiotic
- LB + Spectinomycin
- LB + tetracyclin
- LB + Chloramphenicol
- MacConkey without antibiotic
- MacConkey + Spectinomycin
- MacConkey + Tetracyclin
- MacConkey + Chloramphenicol
Incubation ON 37°C
Measurment
'By Johan'
https://2015.igem.org/Team:Paris_Saclay/Measurement#29th_July
Member present:
- Instructors: Alice
- Students: Coralie, Audrey, Pauline, Johan and Seong-Koo