Team:Paris Saclay/Notebook/July/8
Contents
Wednesday 8th July
Lab Work
Transformation
by Audrey
- BBa_R0051 (2015)
On LB + Ampicillin 100ug/mL
Rehydratation
by Seong Koo, Johan
- BBa_R0051 from different years (2013 and 2014)
Digestion
by Pauline, Coralie
First Digestion:
- BBa_J23101
- BBa_J23106
- BBa_J23117
Mix:
- 10µL of our plasmid with promotor
- 1µL SpeI
- 1µL PstI
- 2µL buffer 10x FastDigest
- 6µL H2O
Second Digestion:
- BBa_K115017
- BBa_I13504
Mix:
- 10µL of our plasmid with gene
- 1µL XbaI
- 1µL PstI
- 2µL buffer 10x FastDigest
Incubation 1h30, 37°C
After this step, we separate the sequence we need from the sequence we don't with electrophoresis
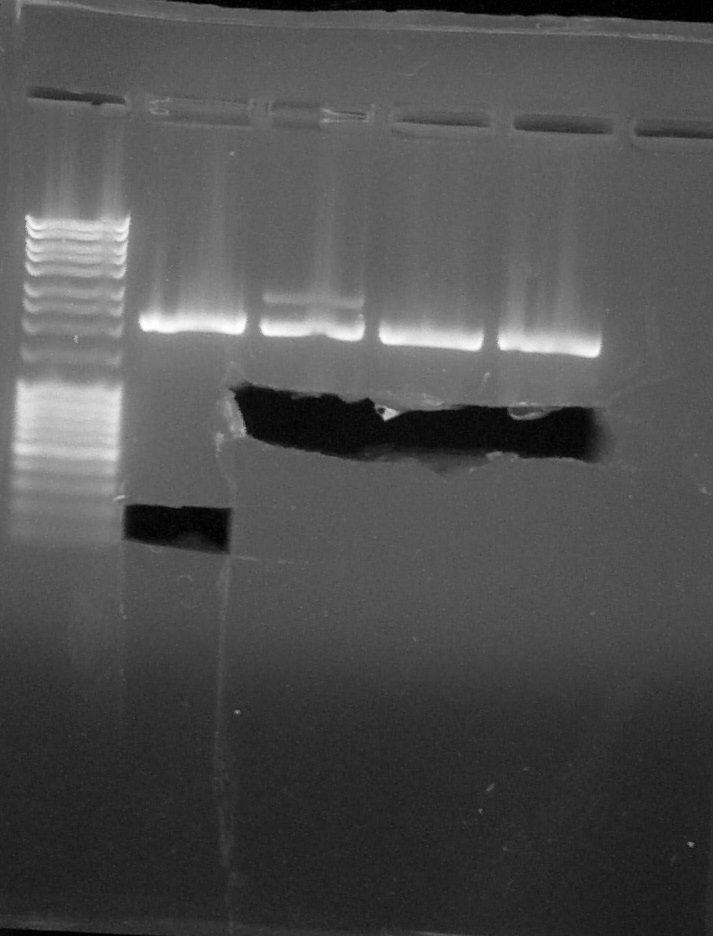
Electrophoresis
by Coralie, Pauline
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5uL of BET Migration 0,06A 80V
- BBa_K115017
- BBa_I13504
We cut our bands with a scalpel
Cut verification, from left to right: 1. DNA Ladder, 2. BBa_K115017, 3. BBa_I13504#1, 4. BBa_I13504#2, 5. BBa_I13504#3, 6. Empty
Purification from agarose gel
by Audrey and Pauline
With the Nucleospin Gel Clean Up kit from Macherey-Nagel
- BBa_K115017
- BBa_I13504
Plasmid extraction
by Coralie
- BBa_K1493504
With Nucleospin Kit
Digestion
by Coralie
- BBa_K1493504
Mix (2tubes):
- 33µL H2O
- 4µL Buffer FastDigest 10x
- 1µL NotI
We use 2µL of plasmid in each tube
Incubation 1h30, 37°C
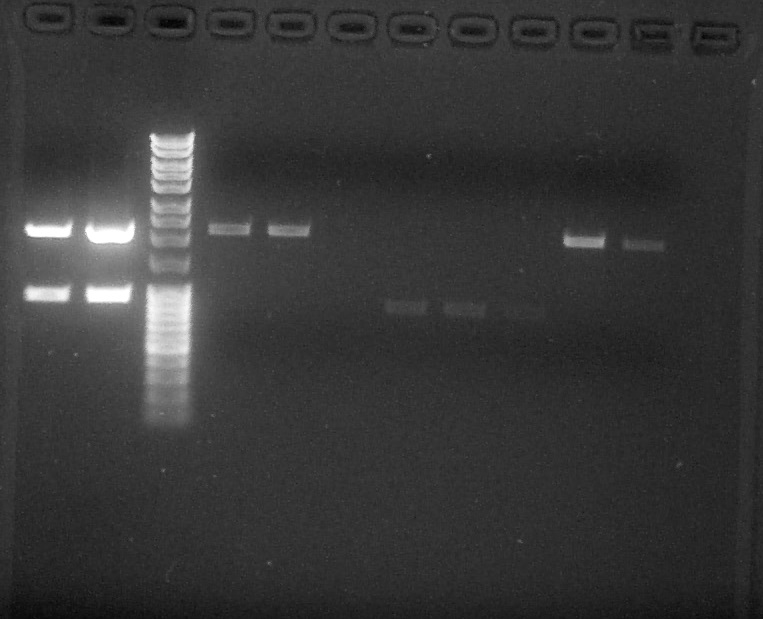
Quantification by electrophoresis
by Pauline
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5uL of BET Migration 0,06A 80V
- Extracted DNA:
- BBa_K115017
- BBa_I13504
- Digested DNA (by SpeI and PstI):
- BBa_J23101
- BBa_J23106
- Digested DNA (by Not I):
- BBa_K1493504
Wells 1-2: Verification/ Wells 4-11: Quantification; from left to right: 1. BBa_K1493504#1, 2. BBa_K1493504#2, 3. DNA Ladder, 4. BBa_J23101#1, 5. BBa_J23101#2, 6. BBa_K115017, 7. BBa_I13504#1, 8. BBa_I13504#2, 9. BBa_I13504#3, 10. BBa_J23106, 11. BBa_J23117, 12. Empty
We can conclude:
- BBa_J23101 #1: 10ng/µL
- BBa_J23101 #2: 13ng/µL
- BBa_I13504 #1 #2 #3: 5ng/µL
- BBa_J23106: 15ng/µL
- BBa_J23117: 8ng/µL
We can't see anything for BBa_K115017
Purification
by Coralie
- BBa_J23101
- BBa_J23106
- BBa_J23117
With Nucleospin Kit
Ligation
by Coralie, Audrey
- BBa_J23117 + BBa_I13504
- 2,5µL J23117
- 15µL I13504
- 1µL T4 DNA Ligase
- 2µL Buffer T4 DNA Ligase 10x
- BBa_J23106 + BBa_I13504
- 1,5µL J23106
- 15,5µL I13504
- 1µL TA DNA Ligase
- 2µL Buffer T4 DNA Ligase 10x
- BBa_J23201 + BBa_I13504
- 2µL J23101
- 14,5µL I13504
- 1µL Ligase
- 2µL buffer 10x
Incubation ON, 16°C
Members present:
- Instructors and advisors: Alice.
- Students: Johan, Seong Koo, Audrey, Coralie, Pauline