Difference between revisions of "Team:Paris Saclay/Notebook/August/25"
m |
m |
||
| Line 9: | Line 9: | ||
Agarose gel 1%, migration 90V | Agarose gel 1%, migration 90V | ||
| − | [[File:ParisSaclay 25.08.15-Purif sur gel.jpg| | + | [[File:ParisSaclay 25.08.15-Purif sur gel.jpg|200px|center]] |
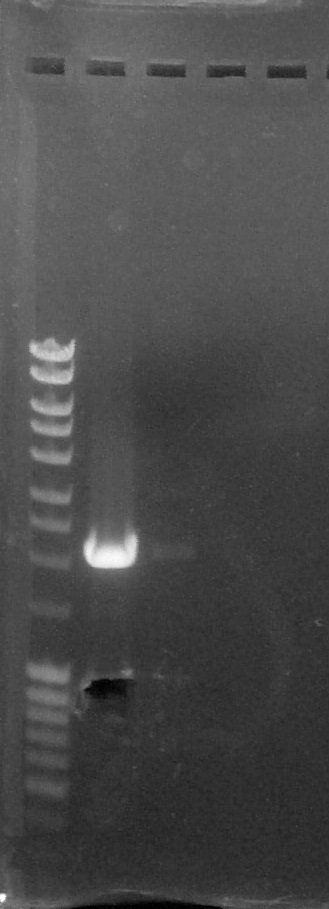
<html><p><i>Verification of gel purification, from left to right: 1. <a href="https://2015.igem.org/File:Paris_Saclay-Ladder.jpg" target="_blank">DNA Ladder</a>, 2. BBa_K1707004#5, 3. BBa_K1707022#1, 4. Empty, 5. Empty</i></p></html> | <html><p><i>Verification of gel purification, from left to right: 1. <a href="https://2015.igem.org/File:Paris_Saclay-Ladder.jpg" target="_blank">DNA Ladder</a>, 2. BBa_K1707004#5, 3. BBa_K1707022#1, 4. Empty, 5. Empty</i></p></html> | ||
We cut corresponding bands with a scalpel. | We cut corresponding bands with a scalpel. | ||
| Line 130: | Line 130: | ||
* 4°C for ever | * 4°C for ever | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
'''Member present:''' | '''Member present:''' | ||
Latest revision as of 23:15, 18 September 2015
Contents
Tuesday 25th August
Lab Work
Electrophoresis purification
by Audrey
- BBa_K1707004 #5 (digested by SpeI and EcoRI)
- BBa_K1707022 #1 (digested by XbaI and PstI)
Agarose gel 1%, migration 90V
Verification of gel purification, from left to right: 1. DNA Ladder, 2. BBa_K1707004#5, 3. BBa_K1707022#1, 4. Empty, 5. Empty
We cut corresponding bands with a scalpel.Purification
by Audrey
- BBa_K1707022 #1
- BBa_K1707023 #1
- BBa_K1707004 #5
- BBa_R0040 #1
With Macherey Nagel purification kit
Quantification
by Audrey
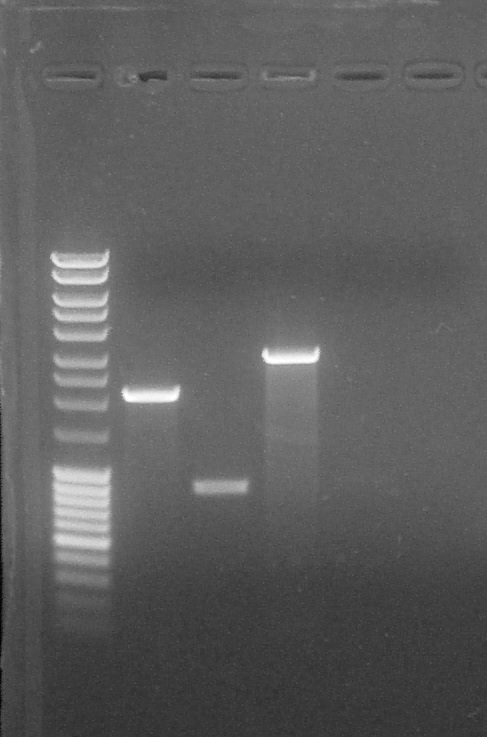
Agarose gel 1%, migration 90V. For each sample:
- 5 µL plasmid
- 5 µL H2O
- 2 µL Ladder 6x
Quantification, from left to right: 1. DNA Ladder, 2. BBa_R0040 (EcoRI+XbaI), 3. BBa_K1707004#5 (SpeI+EcoRI), 4. BBa_K1707004#5 (SpeI+PstI), 5. BBa_K1707022, 6. BBa_K1707023, 7. Empty, 8. Empty, 9. Empty, 10. Empty
We can conclude:- BBa_K1707022 #1: nothing can be seen
- BBa_K1707023 #1: nothing can be seen
- BBa_K1707004 #5 (SpeI + EcoRI): 20 µg/µL
- BBa_K1707004 #5 (SpeI + PstI): 75 µg/µL
- BBa_R0040 #1: 75 µg/µL
PCR for the Gibson experiment
by Audrey
Amplification Thermometer RNA BBa_K115017
PCR mix for each tube:
- 36,75 µL H2O
- 10 µL Buffer Phusion
- 1 μL dNTP 10mM
- 0,5 μL Forward primer
- 0,5 μL Reverse primer
- 1μL plasmid BBa_K115017 v10
- 0,25 μL DNA Pol Phusion
PCR Cycle:
- 98°C - 30 seconds
- 30 cycles:
- 98°C - 5 seconds
- 69,8°C - 30 seconds
- 72°C - 10 seconds
- 72°C - 10 minutes
- 4°C for ever
Amplification (reverse PCR)
BioBricks with cI and cI857:
- BBa_K1707013
- BBa_K1707019
- BBa_K1707020
- BBa_K1707035
- BBa_K1707036
PCR mix for each tube:
- 36,75 µL H2O
- 10 µL Buffer Phusion
- 1 μL dNTP 10mM
- 0,5 μL Forward primer
- 0,5 μL Reverse primer
- 1μL plasmid (dilution 1/10 or 1/100)
- 0,25 μL DNA Pol Phusion
PCR Cycle:
- 98°C - 30 seconds
- 30 cycles:
- 98°C - 5 seconds
- 65,6°C - 30 seconds
- 72°C - 3 minutes
- 72°C - 10 minutes
- 4°C for ever
Amplification (reverse PCR)
- BBa_K1707021
PCR mix for each tube:
- 36,75 µL H2O
- 10 µL Buffer Phusion
- 1 μL dNTP 10mM
- 0,5 μL Forward primer
- 0,5 μL Reverse primer
- 1μL plasmid (dilution 1/10 or 1/100)
- 0,25 μL DNA Pol Phusion
PCR Cycle:
- 98°C - 30 seconds
- 30 cycles:
- 98°C - 5 seconds
- 65,6°C - 30 seconds
- 72°C - 3 minutes
- 72°C - 10 minutes
- 4°C for ever
Amplification (reverse PCR)
- BBa_K1707027
PCR mix for each tube:
- 36,75 µL H2O
- 10 µL Buffer Phusion
- 1 μL dNTP 10mM
- 0,5 μL Forward primer
- 0,5 μL Reverse primer
- 1μL plasmid (dilution 1/10 or 1/100)
- 0,25 μL DNA Pol Phusion
PCR Cycle:
- 98°C - 30 seconds
- 30 cycles:
- 98°C - 5 seconds
- 62,6°C - 30 seconds
- 72°C - 2 minutes
- 72°C - 10 minutes
- 4°C for ever
Member present:
- Instructors: Claire
- Students: Audrey