Team:Paris Saclay/Notebook/August/18
Contents
Tuesday 18th August
Lab Work
Digestion
by Pauline
Biobricks:
- BBa_K1707035 #1 and #2
- BBa_K1707036 #1 and #2
Mix for each plasmid:
- 2 µL plasmid
- 0,5 µL XbaI
- 0,5 µL PstI
- 1 µL Buffer FastDigest 10x
- 6 µL H2O
Incubation 37°C, 3h
Plasmid extraction
by Pauline
- BBa_K1707022 #1 and #2
- BBa_K1707023 #1 and #2
- BBa_K1707034 #1 and #2
With Sylvie Lautru's Protocol
Electrophoresis
by Pauline
Agarose gel 1%, migration 90V
- BBa_K1707035 #1 and #2
- BBa_K1707036 #1 and #2
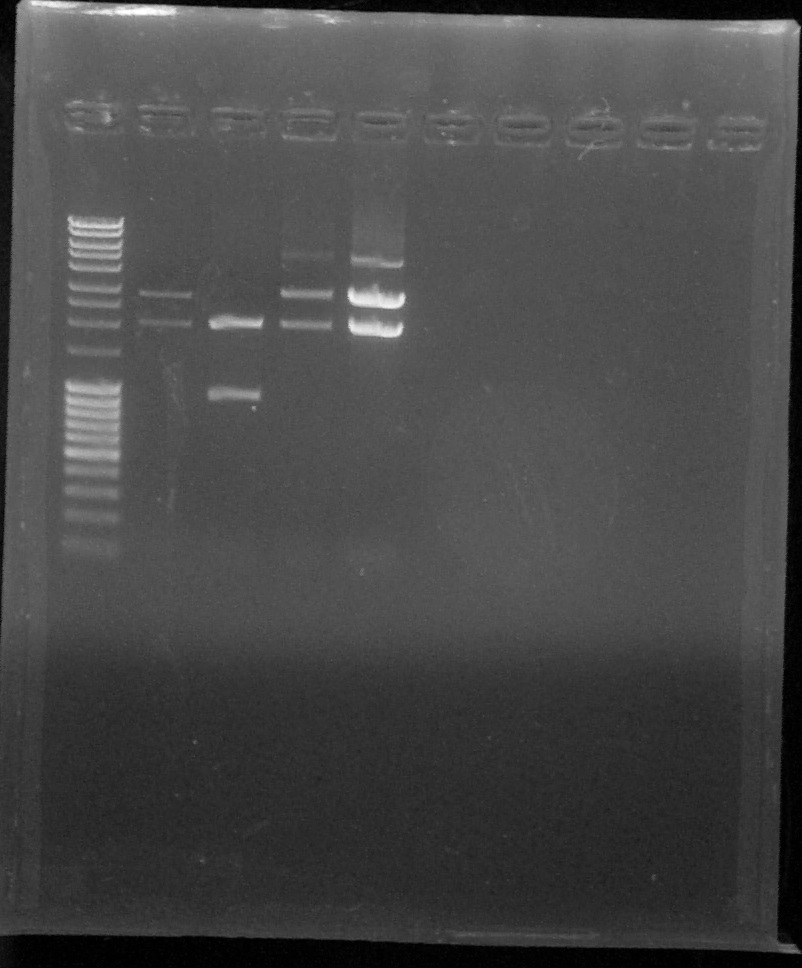
Verification by digestion with XbaI and PstI, from left to right: 1. DNA Ladder, 2. BBa_K1707035#1, 3. BBa_K1707035#2, 4. BBa_K1707036#1, 5. BBa_K1707036#2, 6. Empty, 7. Empty, 8. Empty, 9. Empty, 10. Empty
We can conclude that BBa_K1707035 #1, BBa_K1707036 #1 and #2 are OK, but not BBa_K1707035 #2Inoculation
by Pauline
3 strains: 1320; 1693; 1696
in 10mL LB without antibiotic
PCR
by Audrey
- BBa_K1707000 #2, #3, #4
- BBa_K1707013 #1
- BBa_K1707030 #1
- BBa_K1707019 #1
- BBa_K1707020 #2
- BBa_K1707035 #1
- BBa_K1707036 #1
- BBa_K1707027 #1
- BBa_K1707021 #2
Control +: BBa_K115017 Control -: BBA_J23101+GFP (Interlab study)
Vtot in each tube= 50µL: 2µL plasmid + 48µL mix
Mix for all tubs:
- 476,25 µL H2O
- 150 µL Buffer 5X
- 7,5 µL Forward Primer (iPS43)
- 7,5 µL Reverse Primer (iPS3)
- 15 µL dNTP
- 3,75 µL GoTAQ
- 60 µL MgCl2
Digestion
by Pauline
- BBa_K1707022 #1 and #2
- BBa_K1707023 #1 and #2
- BBa_K1707034 #1 and #2
Mix for each plasmid:
- 2 µL plasmid
- 0,5 µL XbaI
- 0,5 µL PstI
- 1 µL Buffer FastDigest 10x
- 6 µL H2O
Incubation 37°C, 1h
Transplant
by Pauline
Biobrick: BBa_K1707031 clone #7 to #26 In a liquid culture: 5mL LB + 5µL Antibiotic
PCR
by Audrey
Member present:
- Instructors: Claire
- Students: Pauline and Audrey