Difference between revisions of "Team:Paris Saclay/Notebook/July/9"
(→Plasmid Rehydratation :) |
(→Electrophoresis) |
||
| (11 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | = | + | {{Team:Paris_Saclay/notebook_header}} |
| + | =Thursday 9th July= | ||
==Lab Work== | ==Lab Work== | ||
| − | + | ===Transformation=== | |
''by Coralie'' | ''by Coralie'' | ||
Ligation product: | Ligation product: | ||
| − | * | + | * BBa_J23101 + BBa_I13504 |
| − | * | + | * BBa_J23106 + BBa_I13504 |
| − | * | + | * BBa_J23117 + BBa_I13504 |
| − | On LB + | + | On LB + Chloramphenicol 20ug/mL. |
Incubation ON, 37°C | Incubation ON, 37°C | ||
| − | + | ''by Johan and Seong Ko'' | |
| − | + | * BBa_R0051 | |
| − | + | On LB + Ampicillin 100ug/mL. | |
| − | On LB + Ampicillin 100ug/mL | + | |
Incubation ON, 37°C | Incubation ON, 37°C | ||
| − | ===Plasmid Rehydratation | + | ===Plasmid Rehydratation=== |
''by Johan and Audrey'' | ''by Johan and Audrey'' | ||
| − | + | * BBa_S03518 | |
| − | * | + | * BBa_B0030 |
| − | * | + | * BBa_B0015 |
| − | * | + | * BBa_K1399005 |
| − | * | + | |
| − | + | ===Digestion=== | |
''by Pauline and Audrey'' | ''by Pauline and Audrey'' | ||
| − | Plasmid with promotor: | + | Plasmid with promotor: BBa_J23101 |
| − | + | * 10µL of our plasmid with promotor | |
| − | + | * 1µL SpeI | |
| − | + | * 1µL PstI | |
| − | + | * 2µL buffer 10x FD | |
| − | Plasmid with gene: | + | Plasmid with gene: BBa_COO40 and BBa_K115017 |
| − | + | * 10µL of our plasmid with gene | |
| − | + | * 1µL XbaI | |
| − | + | * 1µL PstI | |
| − | + | * 2µL buffer 10x FD | |
After this step, we separate the sequence we need from the sequence we don't with electrophoresis | After this step, we separate the sequence we need from the sequence we don't with electrophoresis | ||
| − | + | ===Electrophoresis=== | |
''by Pauline and Audrey'' | ''by Pauline and Audrey'' | ||
| − | Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0, | + | Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET |
| − | Migration 0,06A 80V | + | Migration 0,06A 80V |
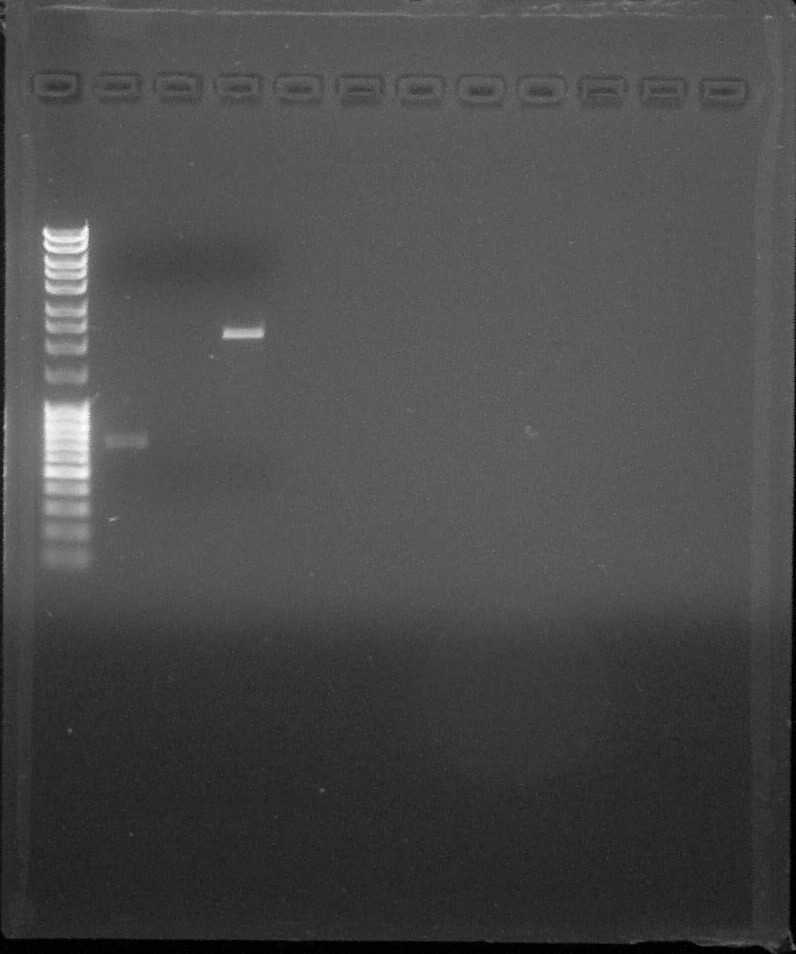
| − | ==== | + | [[File:ParisSaclay_09072015_-_Quantification.jpg|300px|center]] |
| + | <html><i><p>Quantification, from left to right: 1. <a href="https://2015.igem.org/File:Paris_Saclay-Ladder.jpg" target="_blank">DNA Ladder</a>, 2. BBa_C0040, 3. BBa_K115017, 4. BBa_J23101, 5. Empty, 6. Empty, 7. Empty, 8. Empty, 9. Empty, 10. Empty, 11. Empty, 12. Empty</i></p></i></html> | ||
| + | |||
| + | ===Quantification=== | ||
''by Pauline and Audrey'' | ''by Pauline and Audrey'' | ||
| + | |||
| + | Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET | ||
| + | Migration 0,06A 80V | ||
We quantify with the electrophoresis gel: | We quantify with the electrophoresis gel: | ||
| − | + | * BBa_C0040: 10ng/µL | |
| − | + | * BBa_J23101: 20ng/µL | |
| − | + | * BBa_K115017: we don't see enough of fluorescent to quantify the biobrick. We decide to amplify this one by PCR. | |
'''Members present:''' | '''Members present:''' | ||
* Instructors and advisors: Alice. | * Instructors and advisors: Alice. | ||
* Students: Johan, Seong Koo, Audrey, Coralie, Pauline | * Students: Johan, Seong Koo, Audrey, Coralie, Pauline | ||
| + | {{Team:Paris_Saclay/notebook_footer}} | ||
Latest revision as of 21:14, 18 September 2015
Contents
Thursday 9th July
Lab Work
Transformation
by Coralie
Ligation product:
- BBa_J23101 + BBa_I13504
- BBa_J23106 + BBa_I13504
- BBa_J23117 + BBa_I13504
On LB + Chloramphenicol 20ug/mL. Incubation ON, 37°C
by Johan and Seong Ko
- BBa_R0051
On LB + Ampicillin 100ug/mL. Incubation ON, 37°C
Plasmid Rehydratation
by Johan and Audrey
- BBa_S03518
- BBa_B0030
- BBa_B0015
- BBa_K1399005
Digestion
by Pauline and Audrey
Plasmid with promotor: BBa_J23101
- 10µL of our plasmid with promotor
- 1µL SpeI
- 1µL PstI
- 2µL buffer 10x FD
Plasmid with gene: BBa_COO40 and BBa_K115017
- 10µL of our plasmid with gene
- 1µL XbaI
- 1µL PstI
- 2µL buffer 10x FD
After this step, we separate the sequence we need from the sequence we don't with electrophoresis
Electrophoresis
by Pauline and Audrey
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET Migration 0,06A 80V
Quantification, from left to right: 1. DNA Ladder, 2. BBa_C0040, 3. BBa_K115017, 4. BBa_J23101, 5. Empty, 6. Empty, 7. Empty, 8. Empty, 9. Empty, 10. Empty, 11. Empty, 12. Empty
Quantification
by Pauline and Audrey
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET Migration 0,06A 80V We quantify with the electrophoresis gel:
- BBa_C0040: 10ng/µL
- BBa_J23101: 20ng/µL
- BBa_K115017: we don't see enough of fluorescent to quantify the biobrick. We decide to amplify this one by PCR.
Members present:
- Instructors and advisors: Alice.
- Students: Johan, Seong Koo, Audrey, Coralie, Pauline