Difference between revisions of "Team:Paris Saclay/Measurement"
m |
m |
||
| Line 10: | Line 10: | ||
were cloned in the reference plasmid: PSB1C3 at [https://2015.igem.org/Team:Paris_Saclay/Notebook/July/7 7th July]. Controls used are the same constructions, without the BBa_I13504. | were cloned in the reference plasmid: PSB1C3 at [https://2015.igem.org/Team:Paris_Saclay/Notebook/July/7 7th July]. Controls used are the same constructions, without the BBa_I13504. | ||
| − | ===Preparation of the constructions=== | + | ====Preparation of the constructions==== |
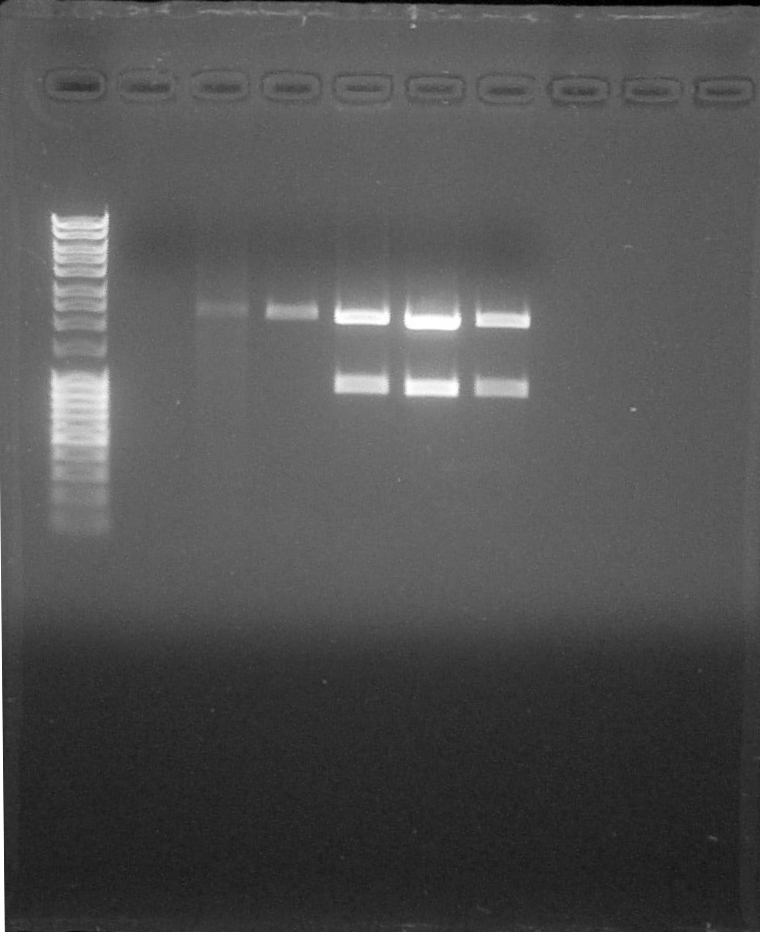
[[File:ParisSaclay_16.07.15_-_digestion_vérif.jpg|250px|right]] | [[File:ParisSaclay_16.07.15_-_digestion_vérif.jpg|250px|right]] | ||
All constructs used were transformed into DH5α strain at [https://2015.igem.org/Team:Paris_Saclay/Notebook/July/16#Digestion 16th July]. First of all, we rehydrated the different BioBricks and transform them into cells. After this step we used the standard assembly protocol we inserted BBa_I13504 as suffix. The different DNA fragments containing promoters were cut with ''SpeI'' and ''PstI'' enzymes, and BBa_I13504 with ''SpeI'' and ''XbaI''. We ligateed them using T4 DNA Ligase. | All constructs used were transformed into DH5α strain at [https://2015.igem.org/Team:Paris_Saclay/Notebook/July/16#Digestion 16th July]. First of all, we rehydrated the different BioBricks and transform them into cells. After this step we used the standard assembly protocol we inserted BBa_I13504 as suffix. The different DNA fragments containing promoters were cut with ''SpeI'' and ''PstI'' enzymes, and BBa_I13504 with ''SpeI'' and ''XbaI''. We ligateed them using T4 DNA Ligase. | ||
The third last wells correspond to our 3 constructs. We observe 2 bands at 2000bp and 900bp corresponding to the expected size for the digested fragments. The identity of our 3 constructs was confirmed by [https://2015.igem.org/Team:Paris_Saclay/Notebook/July/16#Electrophoresis electrophoresis]. | The third last wells correspond to our 3 constructs. We observe 2 bands at 2000bp and 900bp corresponding to the expected size for the digested fragments. The identity of our 3 constructs was confirmed by [https://2015.igem.org/Team:Paris_Saclay/Notebook/July/16#Electrophoresis electrophoresis]. | ||
| − | ===Preview=== | + | ====Preview==== |
After taking our different colonies from the LB plate, we put them on UV lamp. The construction with BBa_J23101 had the strongest fluorescence, but the construction with BBa_J23117 shown the same fluorescence as the control. | After taking our different colonies from the LB plate, we put them on UV lamp. The construction with BBa_J23101 had the strongest fluorescence, but the construction with BBa_J23117 shown the same fluorescence as the control. | ||
| Line 22: | Line 22: | ||
<html><span class="anchor" id="protocols"></span></html> | <html><span class="anchor" id="protocols"></span></html> | ||
==Protocols== | ==Protocols== | ||
| − | ===Measuring=== | + | ====Measuring==== |
In first place we used a flux cytometer PARTEC Cyflow CUBE 6 | In first place we used a flux cytometer PARTEC Cyflow CUBE 6 | ||
All controls correspond to the exact constructions without BBa_I13504 | All controls correspond to the exact constructions without BBa_I13504 | ||
| Line 35: | Line 35: | ||
We observe that promoter BBa_J23101 allows a 10 fold induction of fluorescence compared to BBa_J23106. Moreover J23117 has a curve response comparable to the control. | We observe that promoter BBa_J23101 allows a 10 fold induction of fluorescence compared to BBa_J23106. Moreover J23117 has a curve response comparable to the control. | ||
| − | ===TECAN infinite 200 Pro multimode reader=== | + | ====TECAN infinite 200 Pro multimode reader==== |
Test to obtain the best excitation/emission | Test to obtain the best excitation/emission | ||
We observed with flow cytometry results that the maximum of fluorescence is obtained with BBa_J23101+GFP in stationary phase, we used these conditions to setup the experiment. | We observed with flow cytometry results that the maximum of fluorescence is obtained with BBa_J23101+GFP in stationary phase, we used these conditions to setup the experiment. | ||
| Line 82: | Line 82: | ||
<html><span class="anchor" id="notebook"></span></html> | <html><span class="anchor" id="notebook"></span></html> | ||
==Notebook== | ==Notebook== | ||
| − | ===1st July=== | + | ====1st July==== |
Rehydratation: | Rehydratation: | ||
* BBa_I13504 | * BBa_I13504 | ||
| Line 89: | Line 89: | ||
* BBa_J23101 | * BBa_J23101 | ||
| − | ===2nd July=== | + | ====2nd July==== |
Transformation: | Transformation: | ||
* BBa_I13504 | * BBa_I13504 | ||
| Line 96: | Line 96: | ||
* BBa_J23101 | * BBa_J23101 | ||
| − | ===3rd July=== | + | ====3rd July==== |
Liquide culture: | Liquide culture: | ||
* BBa_I13504 | * BBa_I13504 | ||
| Line 103: | Line 103: | ||
* BBa_J23101 | * BBa_J23101 | ||
| − | ===8th July=== | + | ====8th July==== |
| − | ====First Digestion:==== | + | =====First Digestion:===== |
* BBa_J23101 | * BBa_J23101 | ||
* BBa_J23106 | * BBa_J23106 | ||
| Line 115: | Line 115: | ||
* 6µL H2O | * 6µL H2O | ||
| − | ====Second Digestion:==== | + | =====Second Digestion:===== |
* BBa_I13504 | * BBa_I13504 | ||
Mix: | Mix: | ||
| Line 125: | Line 125: | ||
Incubation 1h30, 37°C | Incubation 1h30, 37°C | ||
| − | ===9th July=== | + | ====9th July==== |
| − | ====Transformation:==== | + | =====Transformation:===== |
* BBa_J23101 + BBa_I13504 | * BBa_J23101 + BBa_I13504 | ||
* BBa_J23106 + BBa_I13504 | * BBa_J23106 + BBa_I13504 | ||
| Line 132: | Line 132: | ||
On LB + Chloramphenicol 20ug/mL. Incubation ON, 37°C | On LB + Chloramphenicol 20ug/mL. Incubation ON, 37°C | ||
| − | ===15th July=== | + | ====15th July==== |
| − | ====Liquid culture:==== | + | =====Liquid culture:===== |
* BBa_J23101 + BBa_I13504 | * BBa_J23101 + BBa_I13504 | ||
* BBa_J23106 + BBa_I13504 | * BBa_J23106 + BBa_I13504 | ||
| Line 140: | Line 140: | ||
We can observe from the plate that BBa_J23101 + BBa_I13504 and BBa_J23106 + BBa_I13504 are yellow when we expose them to the UV light. But BBa_J23117 + BBa_I13504 don't seem to be yellow on the UV light. | We can observe from the plate that BBa_J23101 + BBa_I13504 and BBa_J23106 + BBa_I13504 are yellow when we expose them to the UV light. But BBa_J23117 + BBa_I13504 don't seem to be yellow on the UV light. | ||
| − | ===16th July=== | + | ====16th July==== |
| − | ====Digestion:==== | + | =====Digestion:===== |
* BBa_J23101 + BBa_I13504 | * BBa_J23101 + BBa_I13504 | ||
* BBa_J23106 + BBa_I13504 | * BBa_J23106 + BBa_I13504 | ||
* BBa_J23117 + BBa_I13504 | * BBa_J23117 + BBa_I13504 | ||
| − | ====Reaction mix:==== | + | =====Reaction mix:===== |
* Plasmid: 2µL | * Plasmid: 2µL | ||
* EcoRI: 0,5µL | * EcoRI: 0,5µL | ||
| Line 152: | Line 152: | ||
* Buffer FastDigest (10x): 2µL | * Buffer FastDigest (10x): 2µL | ||
* H2O: 15µL | * H2O: 15µL | ||
| − | ====Electrophoresis:==== | + | =====Electrophoresis:===== |
[[File:ParisSaclay_16.07.15_-_digestion_vérif.jpg|250px|right]] | [[File:ParisSaclay_16.07.15_-_digestion_vérif.jpg|250px|right]] | ||
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET Migration 0,06A 80V | Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET Migration 0,06A 80V | ||
| − | ===17th July=== | + | ====17th July==== |
| − | ====New culture on Plate==== | + | =====New culture on Plate===== |
* BBa_J23101 + BBa_I13504 | * BBa_J23101 + BBa_I13504 | ||
* BBa_J23106 + BBa_I13504 | * BBa_J23106 + BBa_I13504 | ||
* BBa_J23117 + BBa_I13504 | * BBa_J23117 + BBa_I13504 | ||
| − | ===23rd July=== | + | ====23rd July==== |
Liquid culture from the 3 stocks | Liquid culture from the 3 stocks | ||
| − | ===24th July=== | + | ====24th July==== |
| − | ====Cytometer==== | + | =====Cytometer===== |
We count 500 000 events Controls: | We count 500 000 events Controls: | ||
* Alone cells | * Alone cells | ||
| Line 181: | Line 181: | ||
Between each test, we do 2 washes with bleach and 2 washes with H2O | Between each test, we do 2 washes with bleach and 2 washes with H2O | ||
| − | ===28th July=== | + | ====28th July==== |
New culture of BBa_J23101/BBa_J23106/BBa_J23117 + BBa_I13504 | New culture of BBa_J23101/BBa_J23106/BBa_J23117 + BBa_I13504 | ||
| − | ====Tecan utilisation:==== | + | =====Tecan utilisation:===== |
we use only LB without chloramphenicol and we suspect a contamination of our samples. | we use only LB without chloramphenicol and we suspect a contamination of our samples. | ||
We depose in inch well 300µL | We depose in inch well 300µL | ||
| Line 200: | Line 200: | ||
We let's run for 20 cycles of 1 hour | We let's run for 20 cycles of 1 hour | ||
| − | ===29th July=== | + | ====29th July==== |
| − | ====Tecan utilisation:==== | + | =====Tecan utilisation:===== |
This time, we use LB and chloramphenicol because the plate has just a protection and we suspect a contamination of our samples. | This time, we use LB and chloramphenicol because the plate has just a protection and we suspect a contamination of our samples. | ||
| Line 219: | Line 219: | ||
We let's run for 20 cycles of 1 hour | We let's run for 20 cycles of 1 hour | ||
| − | ====Flow cytometer==== | + | =====Flow cytometer===== |
We analyse the sample see previously with ODmax and OD 0.4 But we doesn't use the good scale so we will reused it tomorrow | We analyse the sample see previously with ODmax and OD 0.4 But we doesn't use the good scale so we will reused it tomorrow | ||
| − | ===30th July=== | + | ====30th July==== |
| − | ====Flow cytometer==== | + | =====Flow cytometer===== |
We count 500 000 events Controls: | We count 500 000 events Controls: | ||
* LB | * LB | ||
Revision as of 09:53, 15 September 2015
Contents
Interlab study
Introduction
Simultaneously of our projet, we participated at the Interlab Study 2015 the purpose of this study is to collect fluorescent data from three different constructions with the collaboration of different iGEM teams which came from around the world. We worked with E. coli as chassis, and the 3 constructions
- BBa_J23101 + BBa_I13504
- BBa_J23106 + BBa_I13504
- BBa_J23117 + BBa_I13504
were cloned in the reference plasmid: PSB1C3 at 7th July. Controls used are the same constructions, without the BBa_I13504.
Preparation of the constructions
All constructs used were transformed into DH5α strain at 16th July. First of all, we rehydrated the different BioBricks and transform them into cells. After this step we used the standard assembly protocol we inserted BBa_I13504 as suffix. The different DNA fragments containing promoters were cut with SpeI and PstI enzymes, and BBa_I13504 with SpeI and XbaI. We ligateed them using T4 DNA Ligase. The third last wells correspond to our 3 constructs. We observe 2 bands at 2000bp and 900bp corresponding to the expected size for the digested fragments. The identity of our 3 constructs was confirmed by electrophoresis.
Preview
After taking our different colonies from the LB plate, we put them on UV lamp. The construction with BBa_J23101 had the strongest fluorescence, but the construction with BBa_J23117 shown the same fluorescence as the control.
Our bacteria were put in 5mL LB in 25mL glass tube. Kept at 37°C over night with 150rpm (position with angle in Unitron INFORS HT Incubation Shakers. In the morning we dilute our samples to obtain à 0,1 DO (it take one hour to pass all the control in the cytometer). For the first test, it appeared that bacteria cultures were overgrown, and we observed two times the DO max so we obtain a duplicate of our sample.
Protocols
Measuring
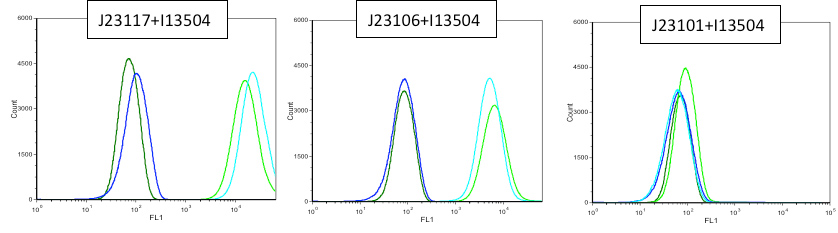
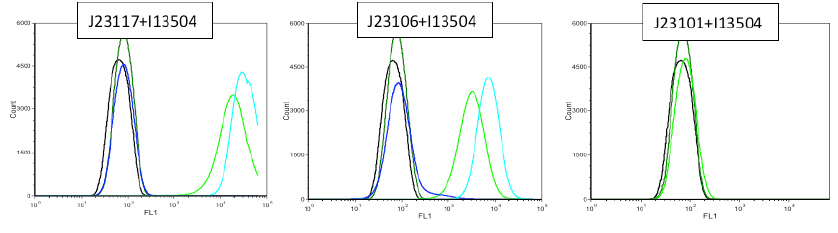
In first place we used a flux cytometer PARTEC Cyflow CUBE 6 All controls correspond to the exact constructions without BBa_I13504 The dark color curves correspond to the controls, the light ones tothe constructions tested. The blue one, with the other blue and the green with the other.
3rd July: The different sample were analyzed at DO max.
30th July: We tested another biological replicate, with DO:0.6 and DOmax, The black picks are LB test.
We observe that promoter BBa_J23101 allows a 10 fold induction of fluorescence compared to BBa_J23106. Moreover J23117 has a curve response comparable to the control.
TECAN infinite 200 Pro multimode reader
Test to obtain the best excitation/emission We observed with flow cytometry results that the maximum of fluorescence is obtained with BBa_J23101+GFP in stationary phase, we used these conditions to setup the experiment.
Excitation 465nm to obtain the best emission wavelength
We fixed the emission wavelength at 520nm and process to obtain the best excitation wavelength
Fluorescence Top Reading
- 460 Nm*
- 520 Nm*
- 9 nm
- 20 nm
- 100 Manual
- 25
- 20 µs
- 0 µs
- 0 ms
Absorbance
- 600 nm
- 9 nm
- 25
- 0 ms
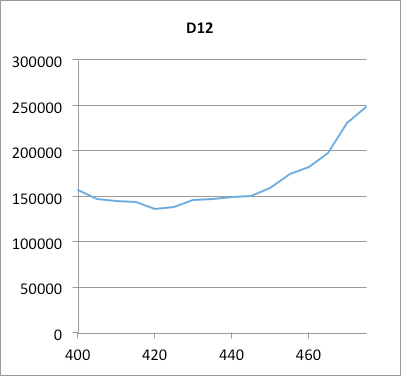
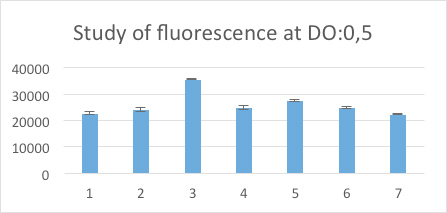
OD600#0,5 (between 0,4 and 0,55) 300 µl of each sample were taken out and put on a 96-well plate (flat transparent bottom, black wall) to measure fluorescence.
Legende:
- LB
- BBa_J23101
- BBa_J23101 + BBa_I13504
- BBa_J23106
- BBa_J23106 + BBa_I13504
- BBa_J23117
We observe a pic of fluorescence with BBa_J23101+BBa_I13504, and a less one with BBa_J23106+BBa_I13504. But with the last one BBa_J23117+BBa_I13504 we observe a result like the negative control.
Results
We see that the construct BBa_J23101 is the strongest of our 3 promoters. BBa_J23106 showed a lowest fold induction response, and the BBa_J23117 showed the same response as the different controls. And this result was obtain with the two different methods (flux cytometer and microplate reader) When the electrophorese was done, we saw that the construct J23117+I13504 was insert in PSB1C3, but it appears that the activity of this promoter was really low or was null.
Notebook
1st July
Rehydratation:
- BBa_I13504
- BBa_J23117
- BBa_J23106
- BBa_J23101
2nd July
Transformation:
- BBa_I13504
- BBa_J23117
- BBa_J23106
- BBa_J23101
3rd July
Liquide culture:
- BBa_I13504
- BBa_J23117
- BBa_J23106
- BBa_J23101
8th July
First Digestion:
- BBa_J23101
- BBa_J23106
- BBa_J23117
Mix:
- 10µL of our plasmid with promotor
- 1µL SpeI
- 1µL PstI
- 2µL buffer 10x FastDigest
- 6µL H2O
Second Digestion:
- BBa_I13504
Mix:
- 10µL of our plasmid with gene
- 1µL XbaI
- 1µL PstI
- 2µL buffer 10x FastDigest
Incubation 1h30, 37°C
9th July
Transformation:
- BBa_J23101 + BBa_I13504
- BBa_J23106 + BBa_I13504
- BBa_J23117 + BBa_I13504
On LB + Chloramphenicol 20ug/mL. Incubation ON, 37°C
15th July
Liquid culture:
- BBa_J23101 + BBa_I13504
- BBa_J23106 + BBa_I13504
- BBa_J23117 + BBa_I13504
We can observe from the plate that BBa_J23101 + BBa_I13504 and BBa_J23106 + BBa_I13504 are yellow when we expose them to the UV light. But BBa_J23117 + BBa_I13504 don't seem to be yellow on the UV light.
16th July
Digestion:
- BBa_J23101 + BBa_I13504
- BBa_J23106 + BBa_I13504
- BBa_J23117 + BBa_I13504
Reaction mix:
- Plasmid: 2µL
- EcoRI: 0,5µL
- PstI: 0,5µL
- Buffer FastDigest (10x): 2µL
- H2O: 15µL
Electrophoresis:
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET Migration 0,06A 80V
17th July
New culture on Plate
- BBa_J23101 + BBa_I13504
- BBa_J23106 + BBa_I13504
- BBa_J23117 + BBa_I13504
23rd July
Liquid culture from the 3 stocks
24th July
Cytometer
We count 500 000 events Controls:
- Alone cells
- Transformed cells by BBa_J23101, BBa_J23106 and BBa_J23117
Our measurements: Transformed cells by
- BBa_J23101 + BBa_I13504
- BBa_J23106 + BBa_I13504
- BBa_J23117 + BBa_I13504
We uses cells in growth phase and stationary phase
Between each test, we do 2 washes with bleach and 2 washes with H2O
28th July
New culture of BBa_J23101/BBa_J23106/BBa_J23117 + BBa_I13504
Tecan utilisation:
we use only LB without chloramphenicol and we suspect a contamination of our samples. We depose in inch well 300µL We analyse the OD and fluorescence's variation (the excitation and emission wave lenght were choose after a scan in the process to obtain the best results) For each sample, we depose twelve time (12x8 plate)
- LB
- Competent cells
- Cells with J23101
- Cells with J23101 + GFP
- Cells with J23106
- Cells with J23106 + GFP
- Cells with J23117
- Cells with J23117 + GFP
We let's run for 20 cycles of 1 hour
29th July
Tecan utilisation:
This time, we use LB and chloramphenicol because the plate has just a protection and we suspect a contamination of our samples. We depose in inch well 300µL We analyse the OD and fluorescence's variation (the excitation and emission wave lenght were choose after a scan in the process to obtain the best results)
For each sample we use 3 different colonies, we depose 2 times each colonies (12x8 plate).
- LB
- Competent cells
- Cells with J23101
- Cells with J23101 + GFP
- Cells with J23106
- Cells with J23106 + GFP
- Cells with J23117
- Cells with J23117 + GFP
We let's run for 20 cycles of 1 hour
Flow cytometer
We analyse the sample see previously with ODmax and OD 0.4 But we doesn't use the good scale so we will reused it tomorrow
30th July
Flow cytometer
We count 500 000 events Controls:
- LB
- Transformed cells by BBa_J23101, BBa_J23106 and BBa_J23117
Our measurements: Cells transformed by
- BBa_J23101 + BBa_I13504
- BBa_J23106 + BBa_I13504
- BBa_J23117 + BBa_I13504
We uses cells in growth phase and stationary phase
Between each test, we do 2 washes with bleach and 2 washes with H2O
We use a less powerful adjustment to see tall the result than the day before.