Difference between revisions of "Team:ETH Zurich/Results"
| Line 228: | Line 228: | ||
[[File:Combinedpromoter74_heatmap.png|thumb|600px|center|Figure x: Heat map showing the fluorescence intensity depending on the concentration of the lactate and IPTG for P<sub>lacUV5-lldR</sub>.]] | [[File:Combinedpromoter74_heatmap.png|thumb|600px|center|Figure x: Heat map showing the fluorescence intensity depending on the concentration of the lactate and IPTG for P<sub>lacUV5-lldR</sub>.]] | ||
<html> | <html> | ||
| − | <p>These results clearly show that one of our combined promoters, P<sub>lac-lldR</sub> (<a href="parts.igem.org/Part:BBa_K1847010">K1847010</a>), reacts in a clear AND gate fashion to a combination of lactate and IPTG. Only in combination, the increase of these two derepressors leads to expression of the reporter GFP. We hereby <b>show that our synthetically engineered hybrid promoter P<sub>lac-lldR</sub> exhibits the desired behavior</b> which will be favourable for the establishment of an AND gate in our <a href="https://2015.igem.org/Team:ETH_Zurich/Glossary#Fold_change_sensor">fold change sensor</a>. The second hybrid promoter we designed, P<sub>lacUV5-lldR</sub> (<a href="parts.igem.org/Part:BBa_K1847011">K1847011</a>), does not show the desired behavior in response to IPTG.</p> | + | <p>These results clearly show that one of our combined promoters, P<sub>lac-lldR</sub> (<a href="parts.igem.org/Part:BBa_K1847010">K1847010</a>), reacts in a clear AND gate fashion to a combination of lactate and IPTG. Only in combination, the increase of these two derepressors leads to expression of the reporter GFP. We hereby <b>show that our synthetically engineered hybrid promoter P<sub>lac-lldR</sub> exhibits the desired behavior</b> which will be favourable for the establishment of an AND gate in our <a href="https://2015.igem.org/Team:ETH_Zurich/Glossary#Fold_change_sensor">fold change sensor</a>. The second hybrid promoter we designed, P<sub>lacUV5-lldR</sub> (<a href="parts.igem.org/Part:BBa_K1847011">K1847011</a>), does not show the desired behavior in response to IPTG. A possible reason for this could be that the optimized LacUV5 promoter is changed in respect to P<sub>lac</sub> in a way that relies on free accessibility of the operator, which might no longer be given if there is LldR interacting with the LldR-operators close by.</p> |
Revision as of 11:20, 18 September 2015
- Project
- Modeling
- Lab
- Human
Practices - Parts
- About Us
Results
We are excited to present a lot of meaningful results, including the characterization of parts of the natural E. coli lldPRD operon on which there is only a very limited amount of information present in the literature up to date. In addition, we attempted to reproduce previously published results on the apoptosis induction in mammalian cancer cell lines using sTRAIL, and tried to validate the Warburg effect for the same cell lines.
For a better understanding of the results of our project, we divided this part into four parts:
Goals
Our main aims during this project were:
- the characterization of our 15 newly designed promoters controlled by the regulator LldR and it's inhibitor L-lactate
- the characterization of 2 newly designed hybrid promoters, combining repression by LldR and LacI
- the investigation of binding of bacteria to apoptotic mammalian cells via Annexin V and phosphatidylserine
- establish a system for display of Annexin V on the E. coli outer membrane
- the design and validation of a tight AND gate with a clear binary behavior
Bacterial sensor
We performed a series of experiments to validate the expression of our constructs as well as the functionality of our system. The results are presented in the following.
Characterization of the LldR promoter
Overview
For the sensing of Lactate inside our system we have a constitutive expression of LldR. LldR is a protein which binds to two operators, operator1 and operator2, that flank the natural LldR promoter. This interaction forms a DNA loop, which does not allow the polymerase to transcribe the respective gene. Once Lactate is added to this system, it sequester LldR which can then no longer repress the promoter, so the gene of interest (LacI in the final system) can be expressed.
An improvement to our system, which was shown by the modeling to be important for enhanced sensitivity to low levels of lactate, is the addition of LldP, a protein which is known to insert into the membrane and increase the importation rate of lactate into the cell.
To find the optimal regulatory system, we designed nine versions of this discribed promoter that would be released from repression by LldR upon addition of Lactate:
Table 1. Design of the promoter regulated by LldR.
| O1-promoter-O2 | promoter-O1-O2 | promoter-O1-spacer-O2 | |
|---|---|---|---|
| J23100 | K1847007 | K1847002 | - |
| J23117 | K1847008 | K1847005 | K1847003 |
| J23118 | K1847009 | K1847004 | K1847006 |
| pLldR | K822000 | - | - |
For characterization of the promoter, we cloned all versions in front of a reporter-GFP. We then measured the sensitivity of our designed promoters to a range of Lactate concentrations by monitoring the intensity of GFP produced by our bacteria expressing constant LldR along with the nine reporter plasmids. Further information on the experimental setup can be found here.
We tested the following combinations of plasmids:
- a medium copy plasmid containing our designed lldR-promoters followed by a reporter GFP (lldRO1-J23117-lldRO2-B0034-sfGFP)
- the described medium copy plasmid and a low copy plasmid containing constant lldR (J23118-B0034-lldR-B0015)
- the described medium copy plasmid and a low copy plasmid containing constant lldR and constant lldP (low levels) (J23114-B0032-lldP-lldR-B0015)
- the described medium copy plasmid and a low copy plasmid containing constant lldR and constant lldP (high levels)(J23118-B0034-lldP-lldR-B0015)
For reasons of simplification, in the following we will refer to the genomic levels of LldR without overexpression from a plasmid as "no lldR".
Results
Initial experiments with all nine promoters showed a clear biase of functionality towards the three versions ressembling the architecture of the natural lldR-promoter (data not shown). For furtehr experiments we therefore focussed on these versions:
- natural version for reference BBa_K822000
- engineered PlldR with high promoter strenght BBa_K1847007
- engineered PlldR with medium promoter strenght BBa_K1847009
- engineered PlldR with low promoter strenght BBa_K1847008
We were lucky to establish a collaboration with Stockholm, where they offered to characterize the less favourable designs for us. In the following we will only focus on the four most promising versions.
We continued to characterize our reduced set of promoters by recording dose response curves amongst a broad range of Lactate concentrations. The first thing to test was whether or not our promoters actually respond to lldR as we expected them to, i.e. if GFP intensity would be properly repressed in the presence of lldR. The resulting data is shown in Figure x.
Surprisingly, what we found was not the expected release from the repression of LldR in the presence of Lactate. Indeed, if no LldR is present in the cell, according to the previous model, the levels of fluorescence should be equivalent as when there is high Lactate and LldR. We can clearly observe additional activation of GFP production! Especially for p69 and p71 the levels of normalized fluorescence upon introduction of Lactate into the system containing LldR rises way above levels observed without LldR1. This interesting finding leads us to reinterpret the present model. Instead of having only repression by LldR, it seems that LldR is required for the transcription machinery. LldR would play a dual role as an activator and as a repressor. That made us think that we need to adapt our model to accomodate this interesting and not explored previously finding.
As mentioned above, we then introduced the Lactate symporter lldP into our system. Due to difficulties in cloning, expression of LldP and LldR are coupled in this case, the result of which is rather low levels of LldR in comparison to the experiment shown above in which there is no lldP (see the table below). We are comparing two different levels of lldP-LldR. To estimate which part of the observed changes results from differences in levels of LldR or lldP, respectively, we also designed a different version of LldR where the expression level is more comparable to the versions including lldP.
Table 2: Expression levels of LldR and LldP in our different test constructs. Levels were calculated using the Salis Lab Ribosomoe Binding Site Calculator in combination with the comparative promoter strenght provided with the Anderson promoter collection.
| Construct | Expression of LldR(A. U.) | Expression of LldP(A. U.) |
|---|---|---|
| LldR | 51100 | - |
| Low LldP- lldR | 664 | 8400 |
| High LldP- lldR | 12 | 23.4 |
Comparison of low levels of LldR and LldP, to the second panel, representing high levels of LldR, can suggest either of two things:
- introduction of lldP into the system leads to leakyness due to uptake of possible traces of Lactate in the medium
- the levels of LldR are too low to properly suppress the LldR-promoter even without presence of Lactate
To discriminate these two possibilities we tested the system, as mentionned, with a higher expression of LldR and LldP (see fourth panel). The reduced level of leakyness despite higher levels of lldP suggests that the problem before was not import of Lactate from the medium (which should not be present anyways) but due to insufficient levels of LldR and therefore lack of proper repression in absence of Lactate.
Based on these results we decided to use BBa_K1847008 in our final system since its high ON/OFF ratio allows for high sensitivity and its KM value of 15.27 allows for sensitivity in the range in which we expect the lactate output of our cancer cells to be located. The validation of the reaction to the lactate levels produced by individual mammalian cells is described below. All in all, we designed a promoter which is repressed by LldR and activated by L-Lactate and responds with an increased sensitivity compared to the natural LldR-operator promoter.ok?.
Characterization of the lacI-lldR promoter
Overview
For the establishment of a fold change sensor topology, a gateway integrating a positive and a delayed negative signal is required. For our system we engineered a fusion promoter integrating activation via lactate and repression via LacI. The two signals come together to influence our newly designed hybrid promoter in which we combined the Lac promoter with its operator (lacO) in the LldR operator promoter. We designed two hybrid promoters using different versions of the lac promoter. A closer description of the design process for these promoters can be found in our Part Collection.
We tested both of our synthetic promoters for their reaction and tightness to L-lactate, as well as IPTG. The setup of this experiment was a strain containing the following constructs.
- a reporter GFP under the control of our hybrid promoter (lldRO1-plac-lacO-lldRO2-B0034-sfGFP or lldRO1-plac-lacO-lldRO2-B0034-sfGFP
- constantly expressed lldR and lldP (J23118-B0034-lldP-lldR-B0015)
- constantly expressed LacI (J23109B0034LacI
Results
The promoter responded to both lactate and IPTG, as can be seen in Figure x.
These results clearly show that one of our combined promoters, Plac-lldR (K1847010), reacts in a clear AND gate fashion to a combination of lactate and IPTG. Only in combination, the increase of these two derepressors leads to expression of the reporter GFP. We hereby show that our synthetically engineered hybrid promoter Plac-lldR exhibits the desired behavior which will be favourable for the establishment of an AND gate in our fold change sensor. The second hybrid promoter we designed, PlacUV5-lldR (K1847011), does not show the desired behavior in response to IPTG. A possible reason for this could be that the optimized LacUV5 promoter is changed in respect to Plac in a way that relies on free accessibility of the operator, which might no longer be given if there is LldR interacting with the LldR-operators close by.
Quorum sensing module: influence of AHL degradation
Overview
The basis of quorum sensing is the leaky production of AHL in absence of an external cue which is crucial to allow for basal levels of AHL needed to trigger quorum sensing upon colocalization of several bacteria. Unfortunately, this leakiness interferes greatly with our system, since the main point of a binary signal like we want it, is the tightness in absence of a trigger. We thought of several strategies to deal with this issue. One option was the introduction of AHL degradation which would help us eliminate unspecifically produced AHL. We therfore included constitutive expression of the AHL Lactonase AiiA in our design. To validate our design, we tested the influence of AHL degradation on the sensitivity to a large range of AHL concentrations.
We tested the response to AHL for the following genetic setups:
- a reporter GFP under conrol of Plux (plux-B0034-sfGFP)
- a reporter GFP under conrol of Plux along with constitutive LuxR (plux-B0034-sfGFP-B0012, J23118-B0032-LuxR-B0012)
- a reporter GFP under conrol of Plux along with constitutive LuxR and constitutive AiiA (plux-B0034-sfGFP-B0012, J23118-B0032-LuxR-B0012, J23118-B0034-aiiA)
Results
Our experimetns comparing the reaction of our system to AHL in the presence or absence of AiiA provided us with impressive results for the dose response and apparent K M values:
Figure 3:Here we compared the experimental and fitted curves of AiiA and the K

|

|
||||
|
|
The characterization of AiiA provided by these results indicate that the KM of Plux can be tuned over a big range of concentration by adaption of the expression levels of AiiA, since in our experimental setup we are comparing the absence of AiiA with a very high level of AiiA, leaving a lot of freedom to adaption of the levels of expression.
Upon extensive modeling of our system including the parameters gained from this study we could show that AHL degradation may solve our problem of leakiness of AHL production encountered in our system. However, to overcome the AHL degradation at high levels of AiiA, our cells would have to produce very high levels of AHL as well, which will be impossible in practice. Unfortunately, lowering the levels of AiiA would allow for sensitivity in the desired range of AHL, but will preventthe buildup of a difference in AHL levels encountered by cells colocalized on a cancer cell when compared to E. coli cells floating freely in the bulk. We therefore decided not to rely on AHL degradation for our final system.
Tightness of the AND gate between lactate module and AHL module
Overview
The ultimate goal of our system's setup is to receive a binary signal combining two independent indicators of CTC presence.Results
INP-Annexin V expression
Overview
We conceptualize our Microbeacon system to rely on bacteria binding to cancer cells as a co-localization signal. The solution that we propose is to use Annexin V expressed on the bacterial membrane to bind cancer cells. Annexin V binds to phosphatidylserine, which is a membrane lipid usually found in the inner part of the cell, but in cells undergoing apoptosis it flips to the outer membrane due to the activity of flipases.
Annexin V is a soluble protein with an unknown function. As we want to express Annexin V in the outer membrane we need a protein that will export it. For this purpose we chose INP ((BBa_K523013)) and changed the YFP for a human Annexin V protein optimized for Escherichia coli codon usage (Life Technologies). We used a strong RBS (BBa_B0034), a low expression promoter (BBa_J23114) and a low copy plasmid pSEVA371 to avoid an excessive stress for the cell, as it is a membrane protein.
Results
To test our construct we used:
- J23114-B0032-INP-Annexin V in pSEVA371 (low copy plasmid)expressing RFP in TOP10 cells
- J23114 expressing RFP in TOP10 cells
To test whether our experiment works, we used for colonies with the above description. Then, as a first test, we did a test with beads. We used magnetic beads, which we incubated with antibodies for 30 min and then with the bacteria with different constructs. Unfortunately, we got no results from them. We also did a Western blot using our anti-Annexin V antibody (Affimetrix BMS147) observing no band in the Annexin V weight.
We wondered what could have gone wrong, so we checked with an RBS calculator (Salis Lab) whether the expression would be high enough with our design. There, we realized that Annexin V expression was too low and probably our protein was not being expressed. Using B0032 with INP-Annexin V we were getting 410 a.u., while if we changed the RBS to B0034, we would get 12 000 a.u. Therefore, we changed the RBS of INP-Annexin V and Annexin V to B0034. As can be seen in Figure 5 we can see a band at the expected 35.9 kDa. This confirms the expression of Annexin V in our cells. INP-Annexin V is also expressed, which can be seen with a band at the corresponding 70.4 kDa. To confirm that INP-Annexin V was on the membrane fraction we run both the crude of each sample and the supernatant of the centrifuged sample. Annexin V is present in both fractions, while INP-Annexin V is only visible in the crude fraction (Figure 5), which makes us think that it is in the membrane fraction.
For the visualization of the Western Blot, we were forced to increase the sensitivity of the film a lot, which indicates that our primary antibody is not functioning too well. We justify this statment with the amount of pure Annexin V-Alexa488 we applyed to our blot, from which we expect a stronger siganl than we reveived.
INP-Annexin V externalization
Overview
After proving expression of our Annexin V-INP construct it is also crucial to test ot for it's expression and functionality. We did so by using Protein G coated Dynabeads coated with an anti-Annexin V antibody. The detailed protocol can be found elsewhere.
The following conditions were compared.
Table 3:Conditions compared in Dynabeads experiment testing Annexin V functionality. Controls include non-coated beads, as well as the addition of Annexin V-Alexa488 as positive control instead of bacteria.
| bacteria strain | with antibody coated beads | beads without coating | |
|---|---|---|---|
| INP-Annexin V (high copy) | a | b | c |
| INP-Annexin V (low copy) | d | e | f |
| rfp | g | h | i |
| control: Annexin V-Alexa488 | j | k | l |
After incubation of the antibody coated beads with the respective bacterial strain or control, we observed our beads using the microscope.
Results
First thing we did was to check the positive (Alexa-488 and beads with antibody and Alexa-488) and negative controls (beads without antibody). We found out that there was no green fluorescence in the positive control, confirming again our fear that the anti-Annexin antibody we are using is of inferiour quality.. Neither of our samples presented fluoresncence from the secondary antibody either. We therefore concluded that the antibody was not very effective to detect annexin bound to another molecule. We came back to our Western blot, where we used the same antibody, and saw that there were some unspecific bands at about 55 kDa. Also, the expected bands were very weak.
INP-Annexin V functionality
Overview
A properly expressed and exported Annexin V protein is the first step towards a bacterial apoptosis detection system. However, the validation of the functionality of Annexin V in this construct is also crucial. We therefore tried several approaches to prove functionality of this construct regarding binding to apoptotic cells. For easy monitoring of our bacteria, we introduced an RFP construct into the same plasmid carrying INP-Annexin V. We published this construct in the registry to allow future iGEM teams to use our bacterial apoptosis detector (BBa_K1847015) for their project.
Results and Discussion
The first atempt we took were beads coated in phosphatidyl serine, which would simulate the surface of an apoptotic mammalian cell. Unfortunately, we were not able to detect binding of bacteria to these beads.
However, these results cannot be taken as a total disproval of our system, since it is not clear how well the phosphatidylserine (PS)-coated beads simulate an apoptotic cell. We came up with the following possible explanations as to why this setup might not be ideal:
- It is possible that too high concentrations of PS on the beads interferes with the Annexin V-PS interaction.
- The hydrophobic tales of PS might form clumps if they are not embedded into a membrane, thereby hindering interactions.
- The biotin moiety required to bind PS onto the beads might be positionned on the lipid such that the interaction with Annexin V is no longer possible
But this initial backset could not stop us! We decided to try a more realistic setup, testing the binding of our E. coli cells expressing the INP-Annexin V construct to actual apoptotic mammalian cells. The results of this experiment can be found here.
Experiments involving mammalian cells
Our first step needed to check the viability of our experimental set-up was to confirm the data found in the literature regarding the sensitivity to TRAIL [Thomas, 2008] and the elevated lactate production observed in cancer cells, but not in normal cells.
Sensitivity to TRAIL
Background
Apoptosis can be induced through a protein called TNF-related Apoptosis Inducing Ligand (TRAIL). TRAIL is a transmembrane protein which can be found also in solubilised form [de Bruyne et al, 2013 ] that recognizes the death receptors DR4, DR5, DCR1, and DCR2 [Johnstone et al, 2008] and it has been used in cancer treatment inducing the apoptosis of cancerous cells but with low cytotoxicity for healthy ones [Johnstone et al, 2008; Zhang et al, 2005].
Experiment overview
The first signal in our system is based on making cancer cells apoptotic while healthy cells remain untouched. Due to the properties of TRAIL explained before, we used it for a selective apoptosis induction in cancer cells.
The aim of this experiment was to check if cancer cells effectively undergo apoptosis after .ong the incubation should last.
To test the predisposition of cancer cells to TRAIL-induced apoptosis we chose Jurkat cells, which are an immortalized human T lymphocyte cell line; and HL-60 cells, a line derived from acute myeloid leukemia. For a control with healthy cells we used a murine fibroblast cell line (3T3).
Methods and Results
Our first experiment consisted in incubating Jurkat and 3T3 cells with TRAIL for 4h and 6h (see protocol about apoptosis sensibility). Concentrations of 0, 50 and 100 ng/mL of TRAIL were used with each cell line. A positive control for Jurkat cells was applied by incubating these cells with PMA and ionomycin overnight. After that, FACS analysis was performed. To differentiate between apoptosis and necrosis, PI dye was used to stain necrotic cells. Apoptotic cells were detected by using Annexin V labelled with Alexa 488 (Life Technologies).
Unfortunately, despite doing several trials in which we incremented the number of hours for incubation (up to 48h) and tried different concentrations of TRAIL, we were not able to replicate the results we found in the literature. To see where the problem was, we decided to incubate the cells with 1000 ng/mL of TRAIL over a period of 48 h. If TRAIL was functional, we would expect to see the cells with a different morphology, which would mean that our detection system is not working even though apoptosis was properly induced. If TRAIL was not functional, we expect not to see morphological differences. After the 48h we saw that there were no differences in the morphology of the cells, which lead us to suppose that probably the sTRAIL we bought was not working as expected.
Our next step was to purchase another TRAIL called killerTRAIL (Enzo). To check whether this time our experiments were working, we added 100 ng/mL of TRAIL to Jurkat cells and measured the apoptosis at 2 h and 4 h. We observed that in 2 h apoptosis increased from about 2% of the population in non-treated samples to almost 70% in cells incubated with TRAIL. After 4 h the apoptotic cell number increased up to 75% (see Figure 1). As the number of apoptotic cells is increasing slowly after 2 h, we decided to use this as our standard time for TRAIL incubation.
Once we confirmed that TRAIL was effectively inducing apoptosis in Jurkat cells, we wanted todo the complete experiment, in which we determine the concentration of TRAIL we will use in future experiments and contrast Jurkat cells with 3T3 (a non-cancerous cell line).
Jurkat cells and 3T3 were incubated for 2 h in 0, 10, 50 and 100 ng/mL of TRAIL. Then, we followed the protocol detailed in Experiments. Annexin V was added to detect apoptosis, as it binds to phosphatidylserine which is found in higher proportion in apoptotic cells. To differentiate apoptotic and necrotic cells, PI dye was added. Annexin V and PI were detected by an emission filter at 488 and 561 nm, respectively.
Our data shows that 3T3 are not affected by TRAIL after 2h, whereas Jurkat cells are (as have been shown in the previous experiment). For Jurkat cells we decided to use a concentration of 100 ng/mL, as it allows to have a higher number of apoptotic cells in a relatively short amount of time (2 h).


Figure 3. 3T3 (upper image) and Jurkat cells (lower image) with 0, 10, 50 and 100 ng/mL of TRAIL after 2 h incubation. Annexin V (488 nm) was used to detect apoptotic cells. To differentiate necrotic cells, PI was added (561 nm).
In Figure 3 in the upper image, for 0 and 50 ng/mL one can see that there is no necrotic cells (Q2). This is probably due to a mistake in manipulation, and these sample lacked PI.
Finally, to check if it was also possible to see apoptotic cells labelled with Annexin V in the microscope, we did an experiment with four conditions. One sample had no TRAIL and no Annexin V; the other had no TRAIL and Annexin V; the next had TRAIL and no Annexin V; and the final had both TRAIL and Annexin V.
As we expecteed, the only sample that had fluorescence was the one with both TRAIL and Annexin V.
Lactate production
Experiment overview
A lot of cancer cells ungergo a metabolic transition, called Warburg effect, where they do the aerobic glycolysis to obtain ATP. A subproduct they generate in this kind of energy production id L-Lactate (Vander et al, 2009). Therefore, the excretion of Lactate by cancer and healthy cells is different enough to be used as an indicator of cancer.
First, we wanted to check the how the production rates of lactate would vary in healthy and cancer cells. To this end, we analysed the medium in which our cells of interest had been incubated for a defined time in a defined seeding density. For detection of lactate in a sample, we used a L-lactate kit (Megazyme), in which two enzymatic reactions lead to a measurable concentration of NADH proportional to the Lactate content of the sample, which can then be measured as optical density at 340 nm (OD340). The kit was used applying the provided protocol.
Methods and results
We used Jurkat cells as our cancer cells, 3T3 as our control for healthy cells and we also measured the concentration of lactate in bacteria. We expected the Jurkat cells to produce more lactate than the 3T3 and the bacteria to produce none.
The first problem we came across measuring the absorbance was the phenol red of the medium of the mammalian cells, which absorbs at the same wavelength as the lactate kit. Once the phenol red was removed, there was still another problem: a component of the medium seemed to react with our kit. After a bibliographic search, we decided that removing FBS from the medium was our best chance to get reliable calibration curves and measurements. We also incorporated a centrifugation step after the collection of the sample to remove any cell that could stay in suspension.
In the case of bacteria, we wanted to take into consideration their fast growth compared to mammalian cells, so we measured the OD600 at each time point to have a correct normalization.
Lactate concentrations were first measured per well, and then normalized by the number of cells present in the well (see protocol about the measurement of Lactate in mammalian cells). The results can be seen in Figure 4.
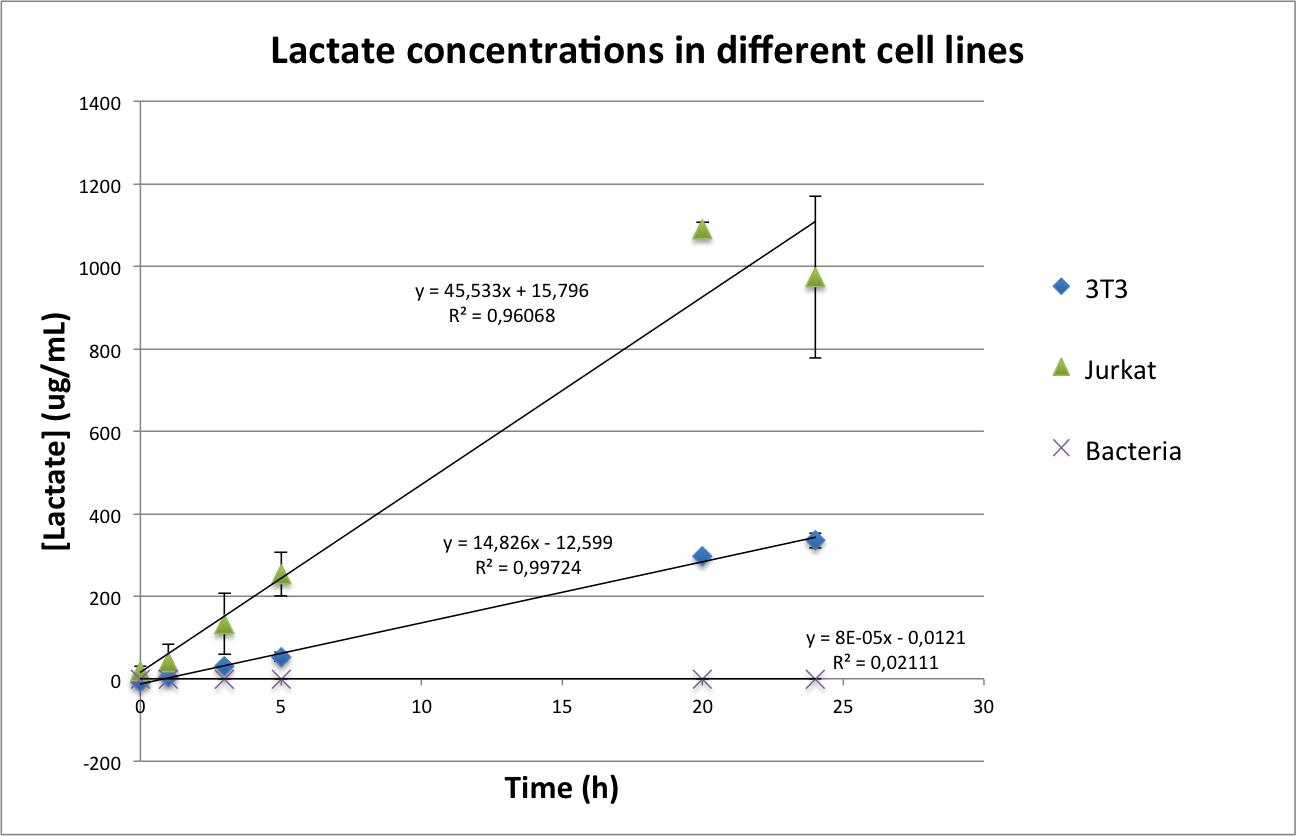

Figure 4. Total concentration of lactate in a well of a 96-well plate (upper image) and normalized values of lactate per cell(lower image).
As can be seen in Figure 4, in the case of bacteria our results confirm the first hypothesis according to which bacteria do not produce lactate. However, they seem to contradict the presence of Warburg effect, as the concentration of lactate per cell is higher in 3T3 than in Jurkat cells. We came across some possible explanations for this phenomenon:
- 3T3 is a murine cell line, while Jurkat cells are a human cell line. It could be possible that basal murine lactate levels are higher than in humans. For checking this hypothesis, we wanted to use human fibroblasts. Unfortunately, none of the labs of our department had any adequate cell line for our purposes and due to time constrains we could not ask other departments.
- 3T3 are adherent cells, while Jurkat cells are non-adherent. Although both cell lines were seeded for not reaching confluency in the next two days it is still possible that the different cell density influenced the metabolism and therefore the lactate production.
In conclusion, we consider that the choice of the 3T3 as a control line was not our best option, but was done due to availability of the cell line and our inexperience using mammalian cells. For future experiments, we want to change our control cell line to produce more conclusive data.
1) For reasons of simplification we are referring to the genomic levels of lldR without overexpression from a plasmid as "no lldR".
Mammalian cells and bacteria combined
Co-culture of mammalian cells with bacteria
Overview
One issue that was concerning us was the possibility that the mammalian cells would behave in unusual ways in presence of bacteria. To exclude this possibility we decided to co-culture mammalian cells with bacteria for 3 h which is the time we expect that our test will last.
We used E. coli expresssing GFP and for the mammalian cell lines we used 3T3, HL-60 and Jurkat lines.
Results
We could observe that after the set time point (3 h) the 3T3 cells incubated with GFP-expressing bacteria had the same morphology as in the control. However, in Jurkat cells, it was possible to see some unspecific binding of bacteria to the cells.

Figure . Co-culture of 3T3 and Jurkat cells without and with bacteria expressing GFP after 3h of incubation.
Detection of apoptotic cells by our engineered bacteria
Bacteria carrying BBa_K1847015 were used here.
Chip
Co-culture of bacteria and 3T3 in the chip
Overview
Thanks to this experiment, we could show that we can introduce a coherent number of bacteria and mammalian cells into the chip. The aim was to obtain single mammalian cells in the chip with 1000 times more bacteria. The loading of the bacteria and mammalian cells is described elsewhere.
To visualize the mixture of cells, we used bacteria expressing RFP and 3T3 P65 cells, whose nuclei display green fluorescence.
Results
We can nicely see that the 3T3 are attaching to the chip.
Lactate detection
To exclude cross reaction with RPMI or DMEM medium, we performed the same experiments as described previously with for our bacteria in these two media.





























