Team:Paris Saclay/Measurement
Measurement
Note
In order to be considered for the Best Measurement Approach award, you must fill out this page.
Contents
1st July
Rehydratation : I13504 J23117 J23106 J23101
07/02
Transformation : I13504 J23117 J23106 J23101
07/03
Liquide culture : I13504 J23117 J23106 J23101
07/08
First Digestion:
BBa_J23101 BBa_J23106 BBa_J23117
Mix:
10µL of our plasmid with promotor 1µL SpeI 1µL PstI 2µL buffer 10x FastDigest 6µL H2O
Second Digestion:
BBa_I13504
Mix:
10µL of our plasmid with gene 1µL XbaI 1µL PstI 2µL buffer 10x FastDigest
Incubation 1h30, 37°C
07/09
Transformation:
BBa_J23101 + BBa_I13504 BBa_J23106 + BBa_I13504 BBa_J23117 + BBa_I13504
On LB + Chloramphenicol 20ug/mL. Incubation ON, 37°C
07/15
Liquid culture:
BBa_J23101 + BBa_I13504 BBa_J23106 + BBa_I13504 BBa_J23117 + BBa_I13504
We can observe from the plate that BBa_J23101 + BBa_I13504 and BBa_J23106 + BBa_I13504 are yellow when we expose them to the UV light. But BBa_J23117 + BBa_I13504 don't seem to be yellow on the UV light.
07/16
Digestion:
BBa_J23101 + BBa_I13504 BBa_J23106 + BBa_I13504 BBa_J23117 + BBa_I13504
Reaction mix:
Plasmid: 2µL EcoRI: 0,5µL PstI: 0,5µL Buffer FastDigest (10x): 2µL H2O: 15µL
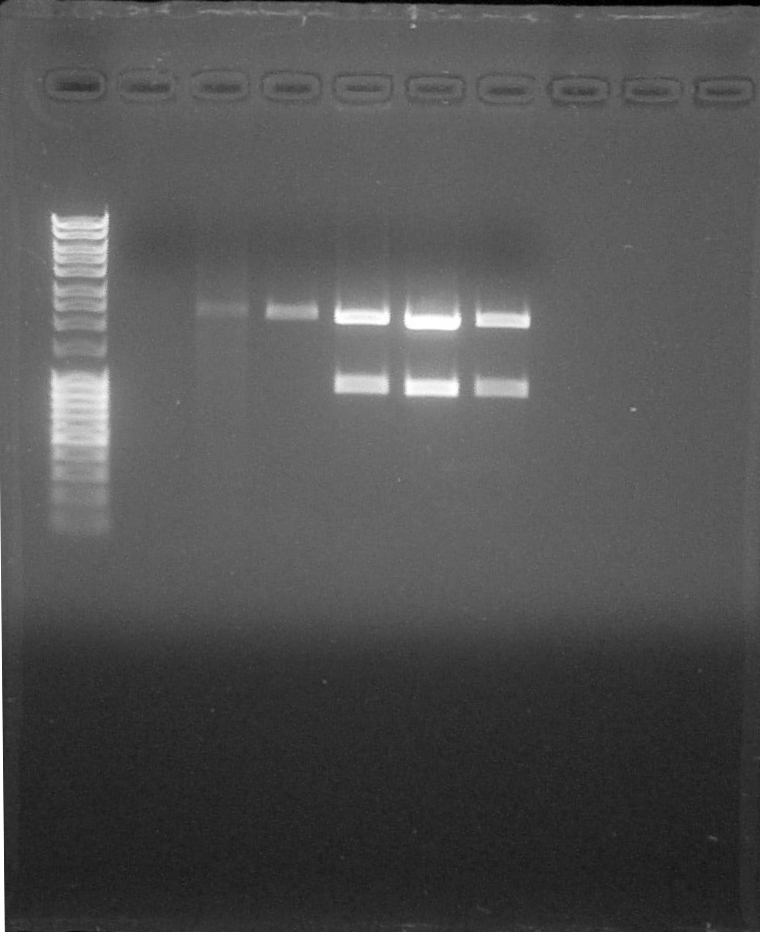
Electrophoresis:
Preparation of Agarose Gel 1%, 0,5g in 50mL of 1X TAE, 0,5µL of BET Migration 0,06A 80V
07/17
New culture on Plate
BBa_J23101 + BBa_I13504 BBa_J23106 + BBa_I13504 BBa_J23117 + BBa_I13504
07/23
Liquid culture from the 3 stocks
07/24
Cytometer
We count 500 000 events Controls:
Cells alones Cells transformed by BBa_J23101, BBa_J23106 and BBa_J23117
Our measurements: Cells transformed by
BBa_J23101 + BBa_I13504 BBa_J23106 + BBa_I13504 BBa_J23117 + BBa_I13504
We uses cells in growth phase and stationary phase
Between each test, we do 2 washes with bleach and 2 washes with H2O
07/28
New culture of BBa_J23101/BBa_J23106/BBa_J23117 + BBa_I13504
Tecan utilisation:
we use only LB without chloramphenicol and we suspect a contamination of our samples. We depose in inch well 300µL We analyse the OD and fluorescence's variation (the excitation and emission wave lenght were choose after a scan in the process to obtain the best results) For each sample, we depose twelve time (12x8 plate)
- LB
- Competent cells
- Cells with J23101
- Cells with J23101 + GFP
- Cells with J23106
- Cells with J23106 + GFP
- Cells with J23117
- Cells with J23117 + GFP
We let's run for 20 cycles of 1 hour
.=07/29=
Tecan utilisation:
This time, we use LB and chloramphenicol because the plate has just a protection and we suspect a contamination of our samples. We depose in inch well 300µL We analyse the OD and fluorescence's variation (the excitation and emission wave lenght were choose after a scan in the process to obtain the best results)
For each sample we use 3 different colonies, we depose 2 times each colonies (12x8 plate)
LB Competent cells Cells with J23101 Cells with J23101 + GFP Cells with J23106 Cells with J23106 + GFP Cells with J23117 Cells with J23117 + GFP
We let's run for 20 cycles of 1 hour
Flow cytometer
We analyse the sample see previously with ODmax and OD 0.4 But we doesn't use the good scale so we will reused it tomorrow
07/30
Flow cytometer
We count 500 000 events Controls:
LB Cells transformed by BBa_J23101, BBa_J23106 and BBa_J23117
Our measurements: Cells transformed by
BBa_J23101 + BBa_I13504 BBa_J23106 + BBa_I13504 BBa_J23117 + BBa_I13504
We uses cells in growth phase and stationary phase
Between each test, we do 2 washes with bleach and 2 washes with H2O
We use a less powerful adjustment to see tall the result than the day before.