Team:Paris Saclay/Notebook/July/23
Contents
Thursday 23th July
Lab Work
Soil experiment
by Audrey, Seong Koo and Johan
Plates observation and count of CFU:
- Strain 1696:
- Dilution 1: 490 CFU
- Dilution 0.1: 67 CFU
- Dilution 0.01: 0 CFU
- Strain 1693:
- Dilution 1: 1308 CFU
- Dilution 0.1: 196 CFU
- Dilution 0.01: 0 CFU
- Strain 1320:
- Dilution 1: 186 CFU
- Dilution 0.1: 35 CFU
- Dilution 0.01: 1 CFU
We take 1g of contaminated soil and treat it like yesterday to spread it on specific plates Incubation ON, 37°C
Digestion
by Coralie
Biobrick: BBa_K1707003 #4 to #9
- Digestion by EcoRI: mix
- 6µL EcoRI
- 6µL Buffer FastDigest 10x
- 36µL H2O
We put 8µL of the mix in each tube + 2µL of our plasmids
- Digestion by EcoRI + PstI: mix
- 6µL EcoRI
- 6µL PstI
- 6µL Buffer FastDigest 10x
- 30µL H2O
We put 8µL of the mix in each tube + 2µL of our plasmids
Incubation 1h30, 37°C
Electrophoresis
by Johan
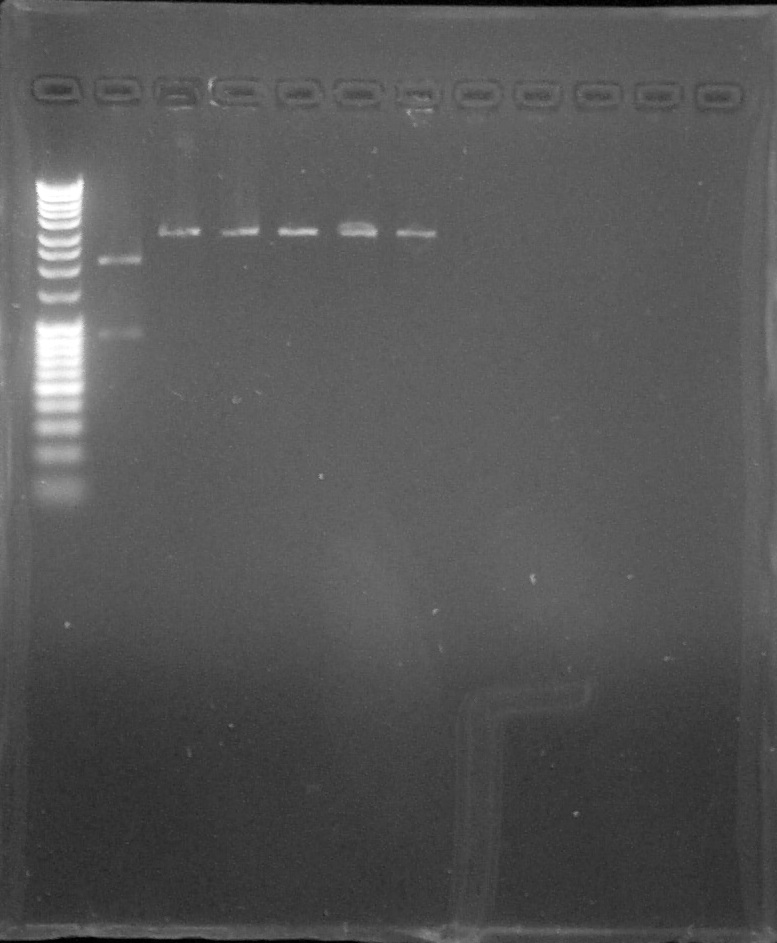
Agarose gel: 1% Migration 80V Biobricks: BBa_K1707003 #4 to #9 digested by EcoRI or EcoRI+PstI
Verification by digestion with EcoRI of BBa_K1707003, from left to right: 1. DNA Ladder, 2. #4 digested by EcorRI and PstI, 3. #5, 4. #6, 5.#7, 6. #8, 7. #9, 8. Empty, 9. Empty, 10. Empty
Verification by digestion with EcoRI and PstI of BBa_K1707003, from left to right: 1. DNA Ladder, 2. #4 digested by EcorRI, 3. #5, 4. #6, 5.#7, 6. #8, 7. #9, 8. Empty, 9. Empty, 10. Empty
We can't conclude anything because we inverted #4 in each gel and we forgot the controlDigestion
by Coralie
Biobricks: BBa_K1707003 #4 and #5 and BBa_B0015
- Digestion by EcoRI: mix
- 3µL EcoRI
- 3µL Buffer FastDigest 10x
- 18µL H2O
We put 8µL of the mix in each tube + 2µL of our plasmids
- Digestion by EcoRI + PstI: mix
- 3µL EcoRI
- 3µL PstI
- 3µL Buffer FastDigest 10x
- 15µL H2O
We put 8µL of the mix in each tube + 2µL of our plasmids
Incubation 1h30, 37°C
Electrophoresis
by Coralie
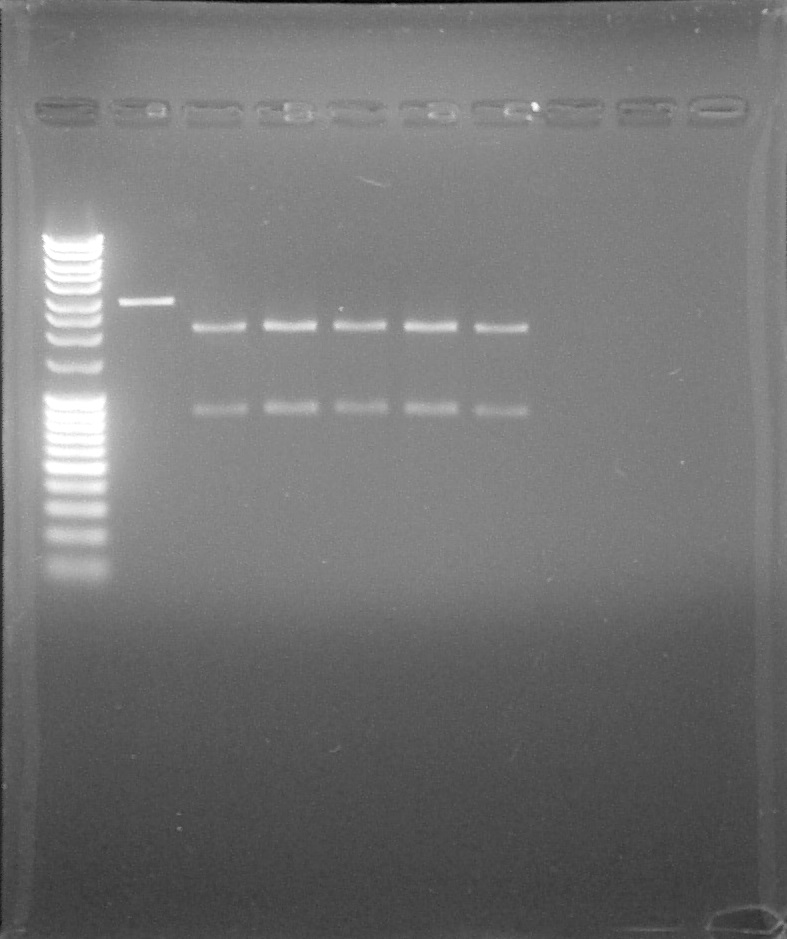
Agarose gel: 1% Migration 80V Biobricks: BBa_K1707003 #4 and #5 and BBa_B0015 digested by EcoRI or EcoRI+PstI
Verification by digestion of BBa_K1707003, from left to right: 1. DNA Ladder, 2. #4 digested by EcorRI, 3. #5 digested by EcorRI, 4. Indicator, 5.#4 digested by EcorRI and PstI, 6. #5 digested by EcorRI and PstI, 7. Indicator, 8. Empty, 9. Empty, 10. Empty
We can confirm the BBa_K1707003 #4 and #5
Gel purification
by Audrey
Biobricks:
- BBa_K1707002
- BBa_K098997
- BBa_C0051
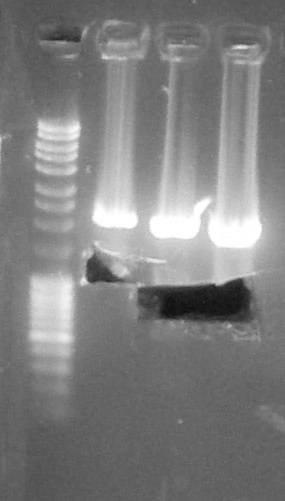
Agarose gel 1%, migration 70V
Verification of gel purification, from left to right: 1. DNA Ladder, 2. BBa_K1707002, 3. BBa_K098997, 4. BBa_C0040
We cut bands with a scalpelPurification
by Audrey
Biobricks:
- BBa_K098997
- BBa_B0015
- BBa_C0051
- BBa_K1707002
- BBa_K1707001
With PCR Clean up/Gel extraction kit from Macherey Nigel
Quantification and Verification
by Audrey
Biobricks:
- Quantification:
- BBa_K098997
- BBa_B0015
- BBa_C0051
- BBa_K1707002
- BBa_K1707001
- Verification:
- BBa_K1707000
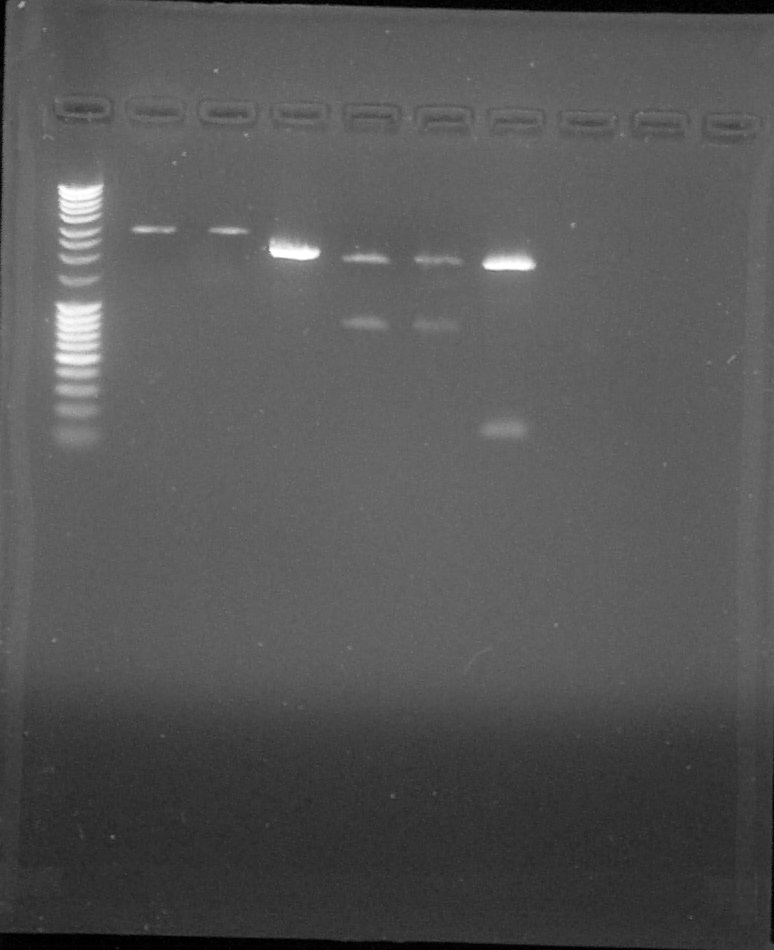
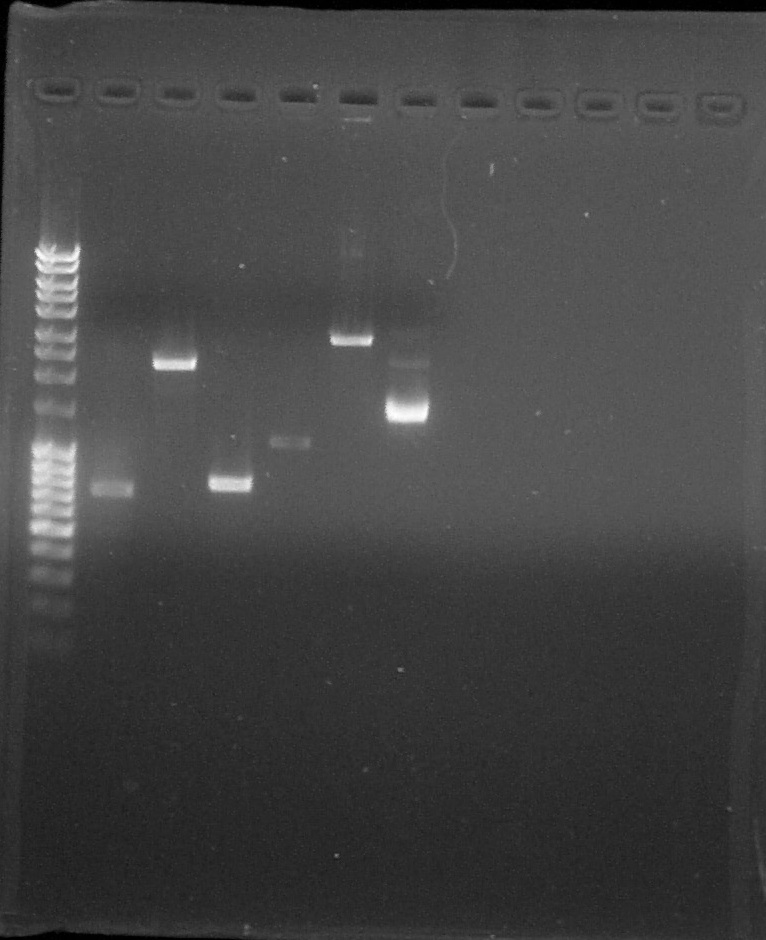
Agarose gel 1% Migration 110V
Wells 2-6: Quantification, Well 7: Verification; from left to right: 1. DNA Ladder, 2. BBa_K098997, 3. BBa_B0015, 4. BBa_C0051, 5. BBa_K1707002, 6. BBa_K1707001, 7. K1707000 plasmid, 8. Empty, 9. Empty, 10. Empty, 11. Empty, 12. Empty
We can conclude that the BBa_K1707000 PCR is ok. So the biobrick is confirmedLigation
by Coralie
- BBa_K1707006
- 3µL BBa_C0051
- 2µL BBa_B0015
- 1µL Ligase
- 1µL Buffer 10x
- 3µL H2O
- BBa_K1707007
- 8µL BBa_K098997
- 2µL BBa_B0015
- 1µL Ligase
- 2µL Buffer 10x
- 7µL H2O
- BBa_K1707005
- 13µL BBa_K1707002
- 2µL BBa_K1707001
- 1µL Ligase
- 2µL Buffer 10x
- 2µL H2O
Incubation ON, 4°C
Member present:
- Instructors:Alice
- Students: Audrey, Coralie, Pauline, Seong Koo, Johan