Team:NTNU Trondheim/Description
Project Description
Background
Diabetes is a metabolic diseases of high level of blood sugar (glucose). People having diabetes can not produce insulin hormone enough to remove excess glucose from the blood. Type 1 diabetes is characterized by a lack of insulin production. Type 2 diabetes is caused by the body’s ineffective use of insulin and it often results from excess body weight and physical inactivity.
Nowadays, around 3% of the norwegian population and 400 million people worldwide suffer from diabetes.
Early diagnosis can be accomplished through blood testing.Treatment of diabetes involves lowering blood glucose and the levels of other known risk factors that damage blood vessels. Healthy lifestyle measures have been shown to be effective in preventing or delaying the onset of type 2 diabetes.
References: http://www.who.int/topics/diabetes_mellitus/.
Motivation
- To create an efficient glucose sensing system in bacteria.
- To create a system that can be encapsulated in alginate, using the capsules as a vector for deploying the bacteria for biomedical applications.
- To find a simpler way than checking the blood sugar level several times a day.
Objectives
- To engineer glucose sensing system in Pseudomonas putida by expressing red fluorescent protein mCherry upon its (glucose) detection (as a proof of concept).
- To encapsulate bacteria in alginate and tailor capsule properties to suit best for our engineered cells and purposes.
- To improve the Matchmaker website.
- To develop a modeling of the glucose biosensor system.
Methods
Genetic engineering
Why did we choose to engineer Pseudomonas putida?
After extensive research we decided to choose Pseudomonas putida over E. coli as transforming organism. Whereas E.coli phosphorylates glucose during its uptake, P. putida …
Glucose metabolism in Pseudomonas putida
Glucose, gluconate and 2-ketogluconate are transported into the cell without being modified (see Figure 1). In the periplasm, glucose can either be transformed into gluconate and subsequently to 2-ketogluconate or it can pass the inner membrane. Approximately half of glucose in the periplasm is directly channeled into the cytosol. The other half is processed via the 2-Ketogluconate peripheral pathway (less than 10% of gluconate produced by glucose is directly passing the inner membrane (del Castillo 2007)). In the cytosol, glucose is transformed via glucokinase (glk) and glucose-6-phosphate 1-dehydrogenase (zfw) into 6-phosphogluconate. 2-Ketogluconate is modified by 2-ketogluconate kinase (kguK) and reductase (kguD), which results in 6-phosphogluconate as well. Finally, 6-phosphogluconate is transformed into 2-keto-3-deoxy-6P-gluconate and subsequently cleaved into pyruvate and glyceraldehyde-3-phosphate (Daddaoua 2010).

The promoters we use
There are four operons whose structural genes are directly concerned with the glucose metabolism. All are negatively controlled, two by the repressor PtxS and the other two by HexR.
The kgu and gad operon, specifically the promoters Pkgu and Pgad contain the operator sequence for the repressor PtxS. It has a DNA binding site as well as a binding site for 2-Ketogluconate. The ptxR and RNA polymerase binding site of Pkgu and Pgad overlap. Thus, PtxR represses gene expression by means of competing with the RNA polymerase for binding. In the presence of 2-Ketogluconate, PtxS changes its conformation and dissociates from the DNA. The RNA polymerase is then able to bind to the DNA and to transcribe the structural genes.
The kgu operon comprises four open reading frames (ORF) coding for kguE, kguK, kguT and kguD (2-ketogluconate epimerase, kinase, transporter and reductase, respectively). The gad operon comprises three ORFs encoding gadC, gadB, and gadA.
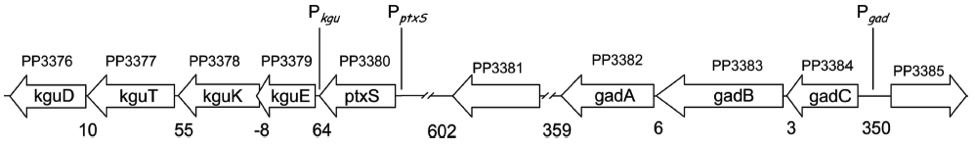
The promoters of the edd and zwf operon contain the operator sequence for the repressor HexR. It is monomeric in solution but assembles to a dimer when binding to DNA. The mechanism of repression is most probably due to inhibition of progression of RNA polymerase. Additionally, HexR has a binding site for 2-keto-3-deoxy-6-phosphogluconate (KDPG). In its presence, the dimeric HexR is released from the DNA and transcription can progress.
Besides the edd gene (encoding 6-phosphogluconate dehydratase), the edd operon comprises the genes glk, gltR2 and gltS (encoding glucokinase and proteins of the glucose transport system, respectively). The zwf operon comprises the genes for zwf, pgl and eda, which all are encoding enzymes for the glucose metabolism as well (Daddaoua 2009).

Our engineered plasmid
Subsequently to the respective promoter our engineered plasmid contains mCherry, a fluorescent protein. Its excitation maximum is at 587 nm, its emission maximum is at 610 nm (www.clonetech.com, 2015). Additionally, the backbone contains KanR (aminoglycoside 3-phosphotransferase), enabling the transformed cell to be resistant against kanamycin.

References
- Daddaoua A, Krell T, Ramos JL (2009) Regulation of glucose metabolism in Pseudomonas. J biol chem 284:21360-21368
- Daddaoua A, Krell T, Alfonso C, Morel B, Ramos JL (2010) Compartmentalized glucose metabolism in Pseudomonas putida is controlled by the PtxS repressor. J Bacteriol 192:4357-4366
- del Castillo T, Ramos JL, Rodriguez-Herva JJ, Fuhrer T, Sauer U, and Duque E (2007) Convergent peripheral pathways catalyze initial glucose catabolism in Pseudomonas putida: genomic and flux analysis. J Bacteriol. 189:5142-5152
- http://www.clontech.com/US/Products/Fluorescent_Proteins_and_Reporters/Fluorescent_Proteins_by_Name/mCherry_Fluorescent_Protein (2015/07/14).
Biochemistry
Modeling
iGEM Matchmaker
Modeling
iGEM Matchmaker
