Team:NTNU Trondheim/Description
Project Description
Background
Diabetes is a metabolic diseases of high level of blood sugar (glucose). People having diabetes can not produce insulin hormone enough to remove excess glucose from the blood. Type 1 diabetes is characterized by a lack of insulin production. Type 2 diabetes is caused by the body’s ineffective use of insulin and it often results from excess body weight and physical inactivity.
Nowadays, around 3% of the norwegian population and 400 million people worldwide suffer from diabetes.
Early diagnosis can be accomplished through blood testing.Treatment of diabetes involves lowering blood glucose and the levels of other known risk factors that damage blood vessels. Healthy lifestyle measures have been shown to be effective in preventing or delaying the onset of type 2 diabetes.
References: http://www.who.int/topics/diabetes_mellitus/.
Motivation
- To create an efficient glucose sensing system in bacteria.
- To create a system that can be encapsulated in alginate, using the capsules as a vector for deploying the bacteria for biomedical applications.
- To find a simpler way than checking the blood sugar level several times a day.
Objectives
- To engineer glucose sensing system in Pseudomonas putida by expressing red fluorescent protein mCherry upon its (glucose) detection (as a proof of concept).
- To encapsulate bacteria in alginate and tailor capsule properties to suit best for our engineered cells and purposes.
- To improve the Matchmaker website.
- To develop a modeling of the glucose biosensor system.
Methods
Genetic engineering
Why did we choose to engineer Pseudomonas putida?
After extensive research we decided to choose Pseudomonas putida over E. coli as transforming organism. Whereas E.coli phosphorylates glucose during its uptake, P. putida …
Glucose metabolism in Pseudomonas putida
Glucose, gluconate and 2-ketogluconate are transported into the cell without being modified (see Figure 1). In the periplasm, glucose can either be transformed into gluconate and subsequently to 2-ketogluconate or it can pass the inner membrane. Approximately half of glucose in the periplasm is directly channeled into the cytosol. The other half is processed via the 2-Ketogluconate peripheral pathway (less than 10% of gluconate produced by glucose is directly passing the inner membrane (del Castillo 2007)). In the cytosol, glucose is transformed via glucokinase (glk) and glucose-6-phosphate 1-dehydrogenase (zfw) into 6-phosphogluconate. 2-Ketogluconate is modified by 2-ketogluconate kinase (kguK) and reductase (kguD), which results in 6-phosphogluconate as well. Finally, 6-phosphogluconate is transformed into 2-keto-3-deoxy-6P-gluconate and subsequently cleaved into pyruvate and glyceraldehyde-3-phosphate (Daddaoua 2010).

The promoters we use
There are four operons whose structural genes are directly concerned with the glucose metabolism. All are negatively controlled, two by the repressor PtxS and the other two by HexR.
The kgu and gad operon, specifically the promoters Pkgu and Pgad contain the operator sequence for the repressor PtxS. It has a DNA binding site as well as a binding site for 2-Ketogluconate. The ptxR and RNA polymerase binding site of Pkgu and Pgad overlap. Thus, PtxR represses gene expression by means of competing with the RNA polymerase for binding. In the presence of 2-Ketogluconate, PtxS changes its conformation and dissociates from the DNA. The RNA polymerase is then able to bind to the DNA and to transcribe the structural genes.
The kgu operon comprises four open reading frames (ORF) coding for kguE, kguK, kguT and kguD (2-ketogluconate epimerase, kinase, transporter and reductase, respectively). The gad operon comprises three ORFs encoding gadC, gadB, and gadA.
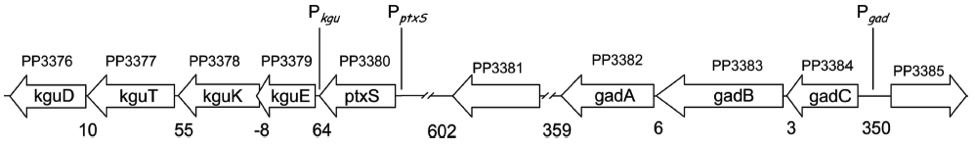
The promoters of the edd and zwf operon contain the operator sequence for the repressor HexR. It is monomeric in solution but assembles to a dimer when binding to DNA. The mechanism of repression is most probably due to inhibition of progression of RNA polymerase. Additionally, HexR has a binding site for 2-keto-3-deoxy-6-phosphogluconate (KDPG). In its presence, the dimeric HexR is released from the DNA and transcription can progress.
Besides the edd gene (encoding 6-phosphogluconate dehydratase), the edd operon comprises the genes glk, gltR2 and gltS (encoding glucokinase and proteins of the glucose transport system, respectively). The zwf operon comprises the genes for zwf, pgl and eda, which all are encoding enzymes for the glucose metabolism as well (Daddaoua 2009).

Our engineered plasmid
Subsequently to the respective promoter our engineered plasmid contains mCherry, a fluorescent protein. Its excitation maximum is at 587 nm, its emission maximum is at 610 nm (www.clonetech.com, 2015). Additionally, the backbone contains KanR (aminoglycoside 3-phosphotransferase), enabling the transformed cell to be resistant against kanamycin.

References
- Daddaoua A, Krell T, Ramos JL (2009) Regulation of glucose metabolism in Pseudomonas. J biol chem 284:21360-21368
- Daddaoua A, Krell T, Alfonso C, Morel B, Ramos JL (2010) Compartmentalized glucose metabolism in Pseudomonas putida is controlled by the PtxS repressor. J Bacteriol 192:4357-4366
- del Castillo T, Ramos JL, Rodriguez-Herva JJ, Fuhrer T, Sauer U, and Duque E (2007) Convergent peripheral pathways catalyze initial glucose catabolism in Pseudomonas putida: genomic and flux analysis. J Bacteriol. 189:5142-5152
- http://www.clontech.com/US/Products/Fluorescent_Proteins_and_Reporters/Fluorescent_Proteins_by_Name/mCherry_Fluorescent_Protein (2015/07/14).
Biochemistry
Cell encapsulation
Cell encapsulation
To show possible applications of our engineered Pseudomonas putida, we plan to encapsulate the cells in alginate. The immobilized cells could be implanted into the body, and function normally while being shielded from the body’s immune response.

Alginate
Alginate is a polymeric biomaterial that can be extracted from marine brown algae (e.g. Laminaria hyperborea), or synthesized by some soil bacteria (some Pseudomonas and Azotobacter species). At NTNU, extensive research has been done for decades on alginate and its many applications - it is even considered to be the national molecule of Norway!
Alginate is a linear polymer consisting of (1-4)-linked β-D-mannuronate (M) and α-L-guluronate (G) residues (Figure 2) (Draget, Moe et al. 2006).

Gelation of Alginate
It is the stretches of G-units that are responsible for gelling of alginate by binding multivalent cations (Draget 2011).The G-units in alginate exist in the 1C4-conformation, and this makes the glycosidic linkages diaxial. This places the rings almost perpendicular to the chain axis, and results in cavities between two or more aligned chains. Two or more G-blocks aligned selectively bind Ca2+ strongly and form junctions in a gel network (also called the egg-box model, as shown in Figure 3). Stretches in the polymer chains consisting of M-blocks and MG-blocks do not bind Ca2+ strongly. (Smidsrød and Moe 2008)

Encapsulation method
Alginate capsules can be made by dropping alginate solution into a gelling solution containing CaCl2, where the alginate will gel instantly when it comes in contact with calcium ions. To encapsulate living cells, they can simply be mixed into the alginate solution prior to gelation. As the alginate solution is very viscous, a capsule generator is necessary to achieve small enough capsules (< 1mm).
The high voltage electrostatic capsule generator (made at the Department of Physics, NTNU) enables the production of alginate microcapsules with a narrow size distribution. The capsule diameter depends on several parameters, such as the applied voltage, needle diameter, the distance between the electrodes and the alginate solution flow rate (Strand, Gåserød et al. 2002). We aim to produce capsules with a diameter of ~200 μm.
References
- Draget KI (2011). Oligomers: Just background noise or as functional elements in structured biopolymer systems? Food Hydrocolloids 25(8): 1963-1965.
- Draget KI, Moe ST, Skjåk-Bræk G, Smidsrød O (2006). Alginates. Food Polysaccharides and Their Applications, CRC Press: 289-334.
- Smidsrød O, Moe ST (2008). Biopolymer chemistry. Trondheim, Tapir Academic Press.
- Strand BL, Gåserød O, Kulseng B, Espevik T, Skjåk-Bræk G (2002). Alginate-polylysine-alginate microcapsules: effect of size reduction on capsule properties. Journal of Microencapsulation 19(5): p. 615-30.
Modeling
The objective of modeling of glucose-detecting bacterial capsules is to reflect the most important dynamics and features of alginate-encapsulated bacterial glucose sensors. The modeling will assist to answer numerous design questions regarding genetic circuits, capsule geometric and physical properties, bacterial density, and predict the performance of the glucose sensor. Agent-based modeling is being conducted to reflect the complexity of the developed system. This approach allows the integration of genetic networks, multiple-physics, growth models, intricate tri-dimensional boundary conditions. The outcomes of the model will be used to assist in design decisions and will be compared with experimental results. Finally, a general analytical and computational framework for bacterial capsule will stem from this project.
iGEM Matchmaker
The iGEM Matchmaker Tool is a website developed by the NTNU iGEM team to make it easy for iGEM teams to collaborate and assist each other. The website has been positively received by the iGEM community in the last years. In 2015, the NTNU Trondheim team has decided to deploy and enhance the existing tool focusing on usability, data mining algorithms, technical uphaul, documentation, and open source practices.
