Team:Georgia State/Project
Project
Mambalgin
Mambalgin E. Coli (BBa_K1800000)
Construct Design

For the design, we first codon optimized the Mambalgin-1 sequence (BBa_K1110003) for Escherichia coli using the IDT codon optimization tool. Restriction sites were then removed from the coding sequence that were iGEM incompatible. We added RFC 10 prefix and suffix and also included a start codon in the beginning of the sequence. Also included in the construct was a prokaryotic RBS (BBa_J61101), IPTG inducible promoter (BBa_R0011), and Myc and 6xHis epitopes. This fusion protein was designed specifically for easy expression in E. coli.
Experimental Design
Our experiment explores the possibility of synthetically producing the analgesic mambalgin-1 protein using E. coli and the yeast Pichia pastoris. Currently, the most effective form of extraction involves milking the venom from Dendroaspis polylepis (black mamba) – a dangerous and costly process. For E. coli, we inserted the mambalgin E. coli construct into the pSB1C3 vector to create a recombinant plasmid. This was then transformed into BL21 expression strain E. coli, which was IPTG induced in liquid cell culture.
Results

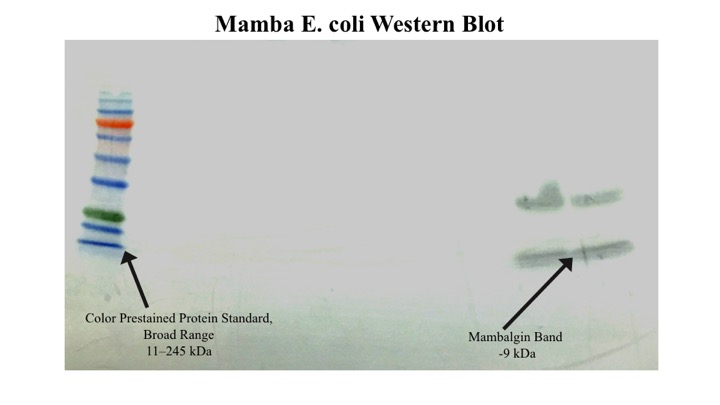
The 2015 GSU iGEM team was able to successfully express and characterize the mambalgin protein in E. coli. After inducing with IPTG overnight, the cell cultures were centrifuged and disrupted with a French press. Mambalgin was isolated using affinity chromatography, utilizing the 6x His tag in the construct. A SDS-PAGE was done, followed by a Ponceau S. and coomassie stain to visualize the proteins. A band indicating a protein of ~9 kda – the size of the mambalgin for E. coli construct – was seen in the eluate fraction.
Mambalgin Yeast (BBa_K1110003)
Construct Design

For the design, we took the coding sequence for mambalgin, removed the TAG stop codon, and added RFC 25 prefix and suffix in Snapgene software to visualize. RFC 25 was used because we were anticipating the use of this part in creating fusion proteins. Additional nucleotides were added before the biobrick prefix and after the biobrick suffix to enable more efficient restriction enzyme cutting at EcoRI and PstI sites. The mambalgin construct was then ordered from IDT and ligated into pSB1C3 backbone.
Using the pGAPzα expression system provided by ThermoFisher Scientific, the GSU team has been working to express the protein in Pichia Pastoris. The pGAPzα vector includes the constitutive glyceraldehyde-3-phosphate dehydrogenase (GAP) promoter, an alpha secretory signal peptide, and Myc and 6x His epitopes. The MCS, between the alpha secretion signal and epitopes, consists of restriction sites which will enable insertion of mambalgin. In-frame insertion into the MCS of pGAPza will be possible after using PCR to modify the construct - removing the RFC 25 prefix and suffix and adding restriction sites and nucleotides.
Experimental Design
Our experiment explores the possibility of synthetically producing the analgesic mambalgin-1 protein using E. coli and the yeast Pichia pastoris. Currently, the most effective form of extraction involves milking the venom from Dendroaspis polyepis (black mamba) – a dangerous and costly process. With Pichia pastoris, we chose to use the shuttle vector pGAPza. This plasmid includes an alpha-factor secretion signal and epitopes enabling secretion of the protein into the culture and easier characterization. To make in-frame insertion into pGAPza possible, the mambalgin for Pichia construct was modified using extension PCR. Using primers, the biobrick prefix and suffix were effectively replaced with restriction sites and nucleotides for correct in-frame insertion. The recombinant pGAPza plasmid was then transformed into Pichia pastoris and grown for expression.
Results
As of yet, we have not successfully been able to express mambalgin in Pichia pastoris.
CBDA Synthase
Construct Design
CBDA Synthase (BBa_K1800003)

CBDA Synthase is also known was cannabidiolic-acid synthase. It does not activate cannabinoid receptors (CB1 and CB2) and it has shown promise in treating many diseases or conditions such as: seizures, cancer, anxiety, pain, and many others. Since the CBDA synthase is 83.9% similar to THCA synthase in its 544-amino acid overlap, the synthesis of CBDA synthase should be similar to THCA synthase (Futoshi et.al, 2007).
Horseradish Peroxidase (BBa_K1800002)

In an effort to show proof of concept for the CBDA synthase portion of the project, the GSU-iGem team is using horseradish peroxidase transformed into agrobacterium then producing transgenic tobacco plants. This part was designed in accordance to RFC 10.
Experimental Design
CBDA Synthase
We will insert the cDNA sequence for CBDA synthase into Agrobacteria using a binary vector system using the pORE vector. This will allow us to transfect tobacco plant tissue with Agrobacteria that code for CBDA synthase creating transgenic tobacco plants that will produce CBDA synthase in their roots. The roots of these plants will then be introduced to CBDA synthase substrate, Cannabigerolic acid, which will be catalyzed to form Cannabidiolic acid. The Cannabidiolic acid will then be separated and heated at 120°C for 20min to form Cannabidiol. Because of the difficulties with producing CBDA synthase, we plan to use the same procedure to produce Horseradish Peroxidase (HRP) in the roots of transgenic tobacco. One of the benefits of this is the bioluminescence that is produced when it catalyzes its substrate.
Results
CBDA Synthase

Though sequencing has not been confirmed yet, we probably ligated the CBDA Synthase part into pSB1C3. We will continue work on this next year.
HRP has been secreted into the media using the pGAP vector in Pichia Pastoris. The secreted protein was concentrated. The expected molecular weight of HRP is 44kDa and is shown on the gel to the right.
