Difference between revisions of "Team:Bielefeld-CeBiTec/Results/HeavyMetals"
| Line 686: | Line 686: | ||
</div> | </div> | ||
</div> | </div> | ||
| + | |||
| + | <p>We tested our nickel sensor with sfGFP as reporter gene, to test the functionality of the system. Moreover we tested different concentrations. The kinetic of our sensors response to different nickel concentrations is shown in figure 3. The first five hours show a strong decrease in fluorescence. After that there is a slight increase in fluorescence. Starting levels of fluorescence are not reached. For better visualization the kinetics of figure 3 are represented as bars in figure 4. A fluorescence level difference for 60 min, 150 min and 650 min is represented.</p> | ||
<p> The data for our nickel sensor show a trend that differs for that of the other sensors. There is no indication for a working sensor <i>in vivo</i> (Figure 3 and 4). There is a fluorescence signal, but it decreases in the first five hours. After reaching a minimum the fluorescence increases slowly. Additionally, there is no difference in fluorescence as response to various nickel concentration. Nickel could influence the cells and thereby caused a precipitation, which could result in decrease of fluorescence. | <p> The data for our nickel sensor show a trend that differs for that of the other sensors. There is no indication for a working sensor <i>in vivo</i> (Figure 3 and 4). There is a fluorescence signal, but it decreases in the first five hours. After reaching a minimum the fluorescence increases slowly. Additionally, there is no difference in fluorescence as response to various nickel concentration. Nickel could influence the cells and thereby caused a precipitation, which could result in decrease of fluorescence. | ||
Revision as of 00:32, 19 September 2015
Heavy Metals
Results

We tested our heavy metal biosensors in Escherichia coli as well as in our cell-free protein synthesis.
Prior to the in vivo characterization, we tested whether the heavy metals have a negative effect on the growth of E. coli.
As can be seen from the figure, we observed no significant difference between the growth in the presence of heavy metals and the controls. This first experiment showed us that in vivo characterization of these sensors is possible. Most cultivations for in vivo characterization were performed in the BioLector. Due to the accuracy of this device, we could measure our samples in duplicates. Subsequently, all functional biosensors were tested in vitro.
Click on the test strip for the results of our biosensor tests in E. coli and in our CFPS:













Arsenic

We choose to work with the chromosomal arsenic operon of E. coli, which was used by the team from Edinburgh in 2006. This operon encodes an efflux pump which confers resistance against arsenic. The expression is controlled by the repressor ArsR, which negatively autoregulates its own expression. AsIII can bind to three cysteine residues in ArsR. The resulting conformational change deactivates the repressor (Chen, Rosen 2014).
By placing a reporter gene downstream of arsR, an arsenic biosensor can be constructed. In this case, both the repressor and the reporter are under the control of the same promoter. In this respect, the arsenic sensor is different from the other heavy metal biosensors we worked with, as their repressors or activators are expressed constitutively. However, the genetic build-up of the arsenic sensor is well-established. Consequently, we decided to keep this deviating design.
Copper
in vivo
We tested an arsenic sensor with mRFP1 as reporter gene in vivo to confirm that the sensor is functional and test whether it is possible to detect the safety limit as defined by the WHO. We observed a reaction approximately five hours after addition of arsenic. The safety limit of 10 µg/L could clearly be distinguished from the negative control and the fluorescence signal increased up to a concentration of 500 µg/L. The signal in the presence of 1000 µg/L was slightly lower than in the presence of 500 µg/L.

in vitro

E. coli is resistant to arsenic because it posseses an efflux pump. The cell extract is not protected by such mechanisms, therefore we tested the effect of arsenic on the synthesis of sfGFP. We observed no significant effect for the relevant safety limits of 10 µg/L and 50 µg/L.

In order to test the arsenic sensor in our cell-free protein synthesis, we cloned a device that contains the arsenic operator between the T7 promoter and sfGFP with our optimized untranslated region (UTR). We tested this device in a cell extract that had been generated from cells expressing the arsenic repressor. We observed an induction when adding arsenic up to a concentration of 1.87 mg/L. As high arsenic concentrations inhibit the performance of the CFPS, we normalized the results to this effect. In the final application, this task is performed by our app.


Compared to the in vivo results, the response to arsenic was relatively small and we measured a high background signal. We assume that this is due to the different construct we used in vitro. This construct had been optimized for our CFPS by exchanging the natural promoter for the T7 promoter and exchanging mRFP1 for our optimized sfGFP. However, we assume that the repression in the presence of ArsR was not effective enough to observe a clear induction. The reason is most likely that the distance between the T7 promoter and the arsenic operator was too large. The distance was a result of our cloning strategy and would likely be suitable for E. coli promoters. However, the T7 promoter requires the operator to be very close for an efficient repression (Karig et al. 2012).
In addition, we performed an experiment in which the arsenic repressor was not present in the reaction from the beginning, but was encoded on a second plasmid. The plasmid concentrations we used had been predicted by our model. In accordance with the aforementioned results, we observed no clear repression and addition of arsenic showed no effect. This experiment is discussed on the Modeling pages.
To summarize
The arsenic sensor is the most prominent example of heavy metal biosensors. Our results confirm that it is possible to detect the safety limit of 10 µg/L arsenic with a cell-based arsenic senosor. The results of the tests in our CFPS indicate that this approach is suitable for the detection of arsenic as well. However, we believe that the genetic construct requires further optimization.
References
Chen, Jian; Rosen, Barry P. (2014): Biosensors for inorganic and organic arsenicals. In Biosensors 4 (4), pp. 494–512. DOI: 10.3390/bios4040494.
Karig, David K.; Iyer, Sukanya; Simpson, Michael L.; Doktycz, Mitchel J. (2012): Expression optimization and synthetic gene networks in cell-free systems. In Nucleic acids research 40 (8), pp. 3763–3774. DOI: 10.1093/nar/gkr1191.
Chromium
Chromium is an essential part of the earth´s crust (Mitchell D. Cohen et al.), but most of it is produced trough industrial uses (Paustenbach et al. 2003). We built a biosensor for the detection of hexavalent (CrVI), because it is toxic and has cancerogenic effects on the human body. An intoxication of chromium can lead to damages of the nervous system. The World Health Organization recommends a limit of 50µg/L in drinking water (Guidelines for drinking-water quality 2011, WHO 2003).
in vivo
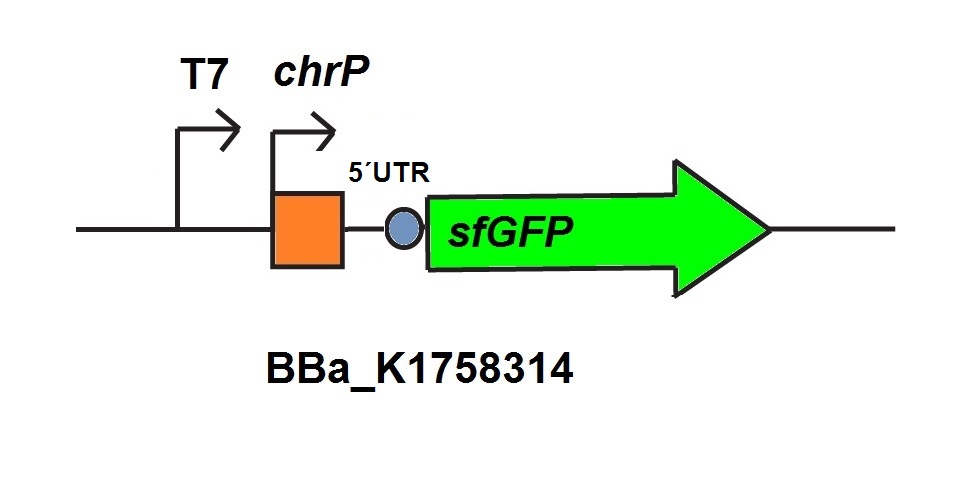
The chromium sensor (BBa_K1758313) was constructed by using the basic construction we showed in Our biosensors. We work with the chromate inducible operon of Ochrobactrum tritici 5bvl1 which enables a resistance for chromium VI and superoxide. For our Sensor we used the Cr (VI) dependent repressor chrB which was introduced by team BIT 2013 (BBa_K1058007), and optimized this sequence for the use in E. coli . The associated chromium responsive promoter is ChrP (introduced by BIT 2013 (BBa_K1058007). For output we used sfGFP and a 5’UTR untranslated region in front of sfGFP to optimize the expression of the reporter protein and increase its fluorescence.
Our sensor for chromium detection consists of ChrB the repressor and the chromate specific promoter ChrP. The promoter is regulated by ChrB, which binds Cr6+-ions. Behind the promoter is a sfGFP for detection of a fluorescence signal.

Our data lead to the conclusion that in a cell based system it is possible to detect chromium. In contrast to our expectations with higher chromium concentrations we got lower fluorescence levels. These observations needed further investigation.Additionally the bar chart showed that the chromium sensor needs a long time to get different fluorescence levels at different chromium concentrations in in vivo experiments. The bar chart showed significant differences between the chromium concentrations after 650 minutes.
in vitro
For the characterization of the chromium sensor with CFPS we used parts differing from that we used in vivo characterization. For the in vitro characterization we used a cell extract out of cells which contain the Plasmid (BBa_K1758310). In addition to that we added Plasmid-DNA of the chromium specific promoter ChrP with 5’UTR-sfGFP under the control of T7-promoter (BBa_K1758314)to the cell extract. The T7-promoter is needed to get a better fluorescence expression.




The test for influence of chromium on the cell extract showed that the influence of chromium at low concentrations is not significant. But the graphic shows that high concentrations of chromium induce fatal damages to the cell extract. At high concentrations the expression of sfGFP is not possible. Therefore the possibility to detect high chromium concentrations needs further investigation.


In addition to the measurements of our chromium sensor in CFPS we measured our chromium inducible promoter with the repressor of team Dundee, which works similar to ours. In contrast to our repressor is only first 15 codons of their repressor are codon-optimized.
References
Guidelines for drinking-water quality (2011). 4th ed. Geneva: World Health Organization, zuletzt geprüft am 20.08.2015.
Mitchell D. Cohen; Biserka Kargacin; Catherine B. Klein; and Max Costa: Mechanisms of Chromium Carcinogenicity and Toxicity, zuletzt geprüft am 19.08.2015.
Paustenbach, Dennis J.; Finley, Brent L.; Mowat, Fionna S.; Kerger, Brent D. (2003): Human health risk and exposure assessment of chromium (VI) in tap water. In: Journal of toxicology and environmental health. Part A 66 (14), S. 1295–1339. DOI: 10.1080/15287390306388.
WHO (2003): Mercury in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality, checked 15.08.15
summarize
Our chromium sensor detects the presence of chromium in vivo, but the outcome differed from our expectations. We would have expected an increase in fluorescence by increasing chromium concentrations. Our in vitro data suggest that these decrease in fluorescence could be explained by chromium’s influence on E. coli which is not reflected in growth but shown by chromium´s influence on the cell extract. Before normalizing the in vitro data the same pattern as in vivo could be observed. After normalization an increase in signal is noticeable. Therefore with optimization our chromium sensor would be compatible to our cell free sensor system.
Copper
There are some possibilities for the contamination of drinking water with copper, for the production of pipes, valves and fittings copper is used (Guidelines for Drinking-water Quality, Fourth Edition ). Copper is an essential trace element for humans, animals and plants, but an overdose can lead to anemia, liver and brain damages (US EPA ORD NCEA Integrated Risk Information System (IRIS) 2014). Additionally high input of copper is associated with aging diseases as Atherosclerosis and Alzheimer’s disease (Brewer 2012). These damages can finally cause death. The World Health Organization recommends a limit of 2 mg/L in drinking water(Guidelines for Drinking-water Quality, Fourth Edition).
in vivo
Our sensor for copper detection consists of CueR a MerR like activator and the copper specific promoter CopAP. The promoter is regulated by CueR, which binds Cu2+-ions. We also used a sfGFP behind the promoter for detection trough a fluorescence signal.
For our copper sensor we used the native operator of cooper homeostasis from E.coli K12. And constructed a part (BBa_K1758324) which was constructed using the basic construction showed in Our biosensors.This operator includes the promoter (copAP), which is regulated by the repressor CueR. This is an important part for the aerobic copper tolerance. In BBa_K1758324 we combined the codon optimized CueR (BBa_K1758320) under the control of a constitutive promoter with CopAP and sfGFP (BBa_K1758321) for measuring output signals. Through the addition of a 5’UTR before the sfGFP we optimized the expression of sfGFP and increased fluorescence.

In vivo we could show that the adding different concentrations of copper has effects on the transcription levels of sfGFP.
The shown data suggest that sensing copper with our device is possible even if the detectable concentrations are higher than the desireble sensitivity limits. Therfore we tested the copper sensor in our in vitro transcription translation approach.
in vitro
For the characterization of the copper sensor with CFPS we used parts differing from that we used in vivo characterization. For the in vitro characterization we used a cell extract out of cells which contain the Plasmid (BBa_K1758320). In addition to that we added Plasmid-DNA of the copper specific promoter copAP with 5’UTR-sfGFP under the control of T7-promoter (BBa_K1758325)to the cell extract. The T7-promoter is needed to get a better fluorescence expression.



As shown above copper has no negative influence on the functuality of our cell extact. Therefore a ralatively stable system for copper sensing is provided.
First tests with specific cell extract and different copper concentrations lead to further tests and normilisations.


In addition to the native promoter, operator device as measured above reporter constructs under the control of T7 promoter were tested.
Fluorescences normalised on coppers influence to the cell extract are shown above.


Compared to the former fluorecence leves the T7 reporter device showed higher levels therefore a reporter device under the control of T7 promoter is more suitable for our CFPS.
After normalising on coppers influcence to the cell extract these differecnces were even more obvious.
References
Background document for development of WHO Guidelines for Drinking-water Quality, checked on 9/9/2015. Copper excess, zinc deficiency, and cognition loss in Alzheimer's disease - Brewer - 2012 - BioFactors - Wiley Online Library. Available online at http://onlinelibrary.wiley.com/doi/10.1002/biof.1005/abstract, checked on 8/28/2015.
US EPA ORD NCEA Integrated Risk Information System (IRIS) (2014): Copper (CASRN 7440-50-8) | IRIS | US EPA. Available online at http://www.epa.gov/iris/subst/0368.htm, updated on 10/31/2014, checked on 9/2/2015.
To summarize
Our copper sensors in vivo data show that detection of different copper concentrations is possible. The fluorescence levels defer clearly between different induction concentrations. As shown above higher copper concentration, higher the fluorescence signal. Therefore the concept of our sensor is functional even if the concentration needed for induction are to high to reach sensitively concerning the WHO guidelines for copper. Our sensor has been tested in vitro as well. For copper we tested our original CopAP construct without a T7 promoter in front of the inducible at first. After realizing that the sensor shows the right tendencies but the general fluorescence is quite low we created an inducible promoter under the control of a T7 promoter to use in CFPS. Fluorescence levels of this device showed the same tendencies as the one without but were higher fluorescence’s, which helps detecting it.
Lead
Lead is one of the most frequently used metals. Therefore, lead is found in many different parts of the environment (World Health Organization (WHO): Fact sheet number 379, Lead poisoning and health). The contamination of drinking water is often caused by obstructed pipes. Long time absorption leads to adverse health effects in most organs in the body (EPA Health Effects: How Lead Affects the body). The main targets are the nervous system, brain and liver. These damages can finally cause death. The World Health Organization recommends a limit of 10 µg/L in drinking water (WHO: Guidelines for Drinking-water Quality, fourth edition (2015)).in vivo
In addition to these we constructed a sensor for lead detection. It consists of PbrR, the repressor, and the lead specific promoter PbrAP. The promoter is regulated by the RcnR, which binds Pb2+-ions. As the former sensors this one encloses a sfGFP for detection via fluorescence.
Our lead sensor consists of parts of the chromosomal lead operon of Cupriavidus metallidurans (figure 2). This operon includes the promoter PbrAP (BBa_K1758332 ), which is regulated by the repressor PbrR. The PbrR belongs to the MerR family, of metal-sensing regulatory proteins, and is Pb2+-inducible. Our sensor system comprises pbrR ( BBa_K1758330 ), which is under the control of a constitutive Promoter and PbrAP and a 5’ untranslated region, which controls the transcription of a sfGFP and increases the fluorescence. Fluorescence implemented by sfGFP protein is the measured output signal (figure 3 and figure 4).

We tested our lead sensor with sfGFP as reporter gene, to test the functionality of the system. Moreover we tested different concentrations. The kinetic of our sensors response to different lead concentrations is shown in figure 3. The first 40 hours show a strong increase in fluorescence. After that the increase in fluorescence is slower. For better visualization the kinetics of figure 3 are represented as bars in figure 4. A fluorescence level difference for 60 min, 150 min and 650 min is represented.
The results of the lead sensor show in vivo no significant differences between the different concentrations (figure 3). But you can see that the decreasing concentrations show a decrease in fluorescence. This biosensor showed the right trend. For using this sensor it has to be optimized. We did not use this sensor in Cell-free-Protein-synthesis, because of the low expression of sfGFP and a lack of time for in vivo tests. In future, it should be characterized with CFPS to show, that this sensor have potential in this system despite in vivo the results. The differences between inductions with various lead concentrations are really slight. Therefore, this sensor needs further optimization which was not possible in the limited time. Nevertheless, there is a fluorescence response to lead. Therefore, this sensor should work as expected. In the future a characterization in CFPS systems would be interesting
References
EPA Health Effects: How Lead Affects the body, checked on 2015-09-17.
WHO Guidelines for Drinking-water Quality fourth edition, checked on 2015-09-09.
WHO lead poisoning and health, fact sheet number 379, reviewed August 2015, checked on 2015-09-17.
To summarize
Our lead sensor was characterized in vivo only. The differences between inductions with various lead concentrations are really slight therefore this sensor needs further optimization which was not possible in this limited time. But as there is a fluorescence response to lead this sensor has the potential work as expected. In the future a characterization in CFPS systems would be interesting.
Mercury
The main sources of mercury exposure are generated through humans. For example mercury contamination can be caused by medical waste (damaged measurement instruments), Fluorescent-lamps, Chlor- Alkali plants and thermal power plants. In the environment, mercury is one of the most toxic elements. Acute effects of a mercury intoxication can range from diseases of the Liver, kidney, gastrointestinal tract, to Neuromuscular and neurological problems. A chronic intoxication of mercury results in kidney changes, changes in the central nervous system and other effects like cancer. The World Health Organization recommends a limit of 6 µg/L in drinking water.
in vivo
One of the already existing sensors we use for our system is the mercury sensor consisting of MerR the activator and the mercury specific promoter PmerT. The promoter is regulated by the MerR, which binds Hg2+-ions. Similar to the former sensors we added a sfGFP for detection via fluorescence.
For our mercury sensor we use parts of the mercury sensor constructed by iGEM team Peking 2010. The parts of iGEM team Peking 2010 consist of the mercury dependent Mer operon from shigella flexneri R100 plasmid Tn21. The expression of the Mer operon is depends on the regulation by MerR its activator and promoter PmerT. For our sensor we used the codon optimized activator (BBa_K1758340), under control of a constitutive promoter, of iGEM Peking 2010 (BBa_K346001). Additionally to the activator of Peking 2010 we used the specific promoter PmerT (BBa_K346002) from this team. For our sensor we added a 5’UTR behind this promoter to increase the fluorscence of the used reporter protein sfGFP.

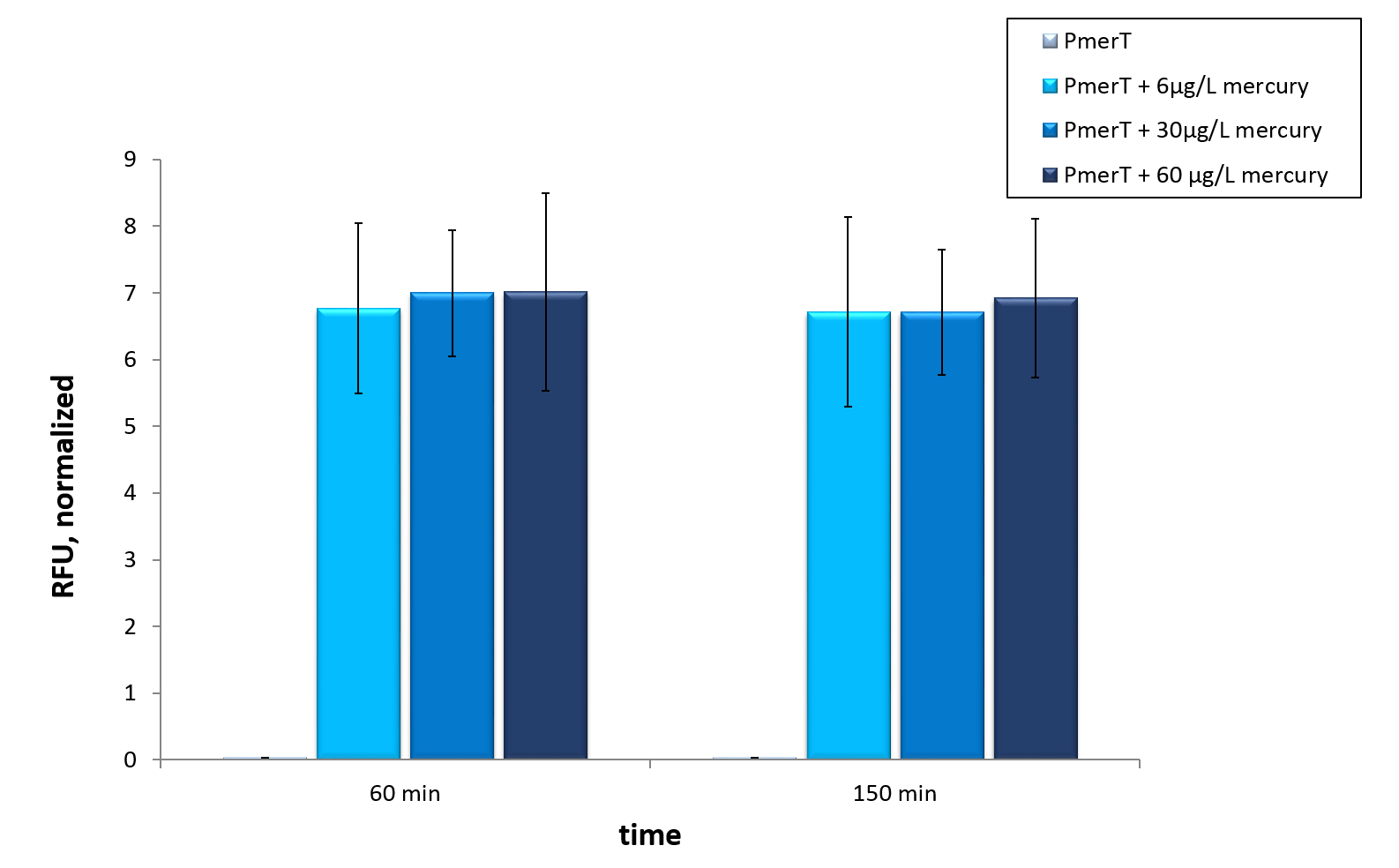
In vivo data show a highly significant, well working sensor which even reacts to concentrations which are mentioned as drinking water guidelines by the WHO.
The mercury detection was measured during the cultivation of E. coli KRX at 37 °C. The strain contains the plasmid with the activator MerRunder the control of a constitutive promoter and the specific promoter with operator site which reacts to the activator with bound Hg2+-ions. The specific promoter is in front of sfGFP for measurment , so the mercury in the medium is detected directly.In vivo this sensor devise shows a fast answer to occurrence of his heavy metal contrary to the other sensor systems in vivo.
Therefore we tested our sensor in vitro to check if an already functioning highly optimized sensor provides required data for guideline detections
in vitro
For the characterization of the mercury sensor with CFPS we used parts differing from that we used in vivo characterization. For the in vitro characterization we used a cell extract out of cells which contain the Plasmid ( BBa_K1758340). In addition to that we added Plasmid-DNA of the copper specific promoter PmerT with 5’UTR-sfGFP under the control of T7-promoter ( BBa_K1758344)to the cell extract. The T7-promoter is needed to get a better fluorescence expression.




To summarize
Our mercury sensor works well in vivo as data show. There is a clearly noticeable increase in fluorescence after induction with mercury. Even the WHO guideline is measurable. This well working sensor was tested in in vitroas well. Taken data suggest, that maximal output is reached at concentrations of 6µg/L, which represent the former mentioned WHO guideline. With optimization a detection of even lower concentrations could be possible in vitro.
Nickel
Naturally nickel occurrences are quite low and rare. In Germany most drinking water pollutions by nickel take place in the last meters of the plumbing system. The reason for these pollutions are wrong tap ware. There may be higher concentrations in drinking-water in special cases of release from natural or industrial nickel deposits. Higher nickel concentrations in water can lead to dermatitis as itching of fingers and other parts of the body through long term skin contact. The World Health Organization (WHO) recommends a limit of 70 µg/L in drinking water.
in vivo
We aimed to construct a sensor for nickel detection. It consists of rcnR the repressor and the nickel specific promoter prcnA. The promoter is regulated by the RcnR, which binds Ni2+-ions. As the former sensors this one encloses a sfGFP for detection via fluorescence.
Our nickel biosensor consists of parts of the rcn-operon from E. coli, which encodes a nickel- and cobalt-efflux system. This system is highly sensitive to nickel. In absence of nickel or cobalt RcnR binds to the operator and inhibits the nickel responsive promoter. With Ni2+-ions present the repression of the promoter prcnA will be reversed, because the repressor RcnR binds Ni2+ ions and cannot attach to the DNA. For our biosensor we construct the part BBa_K1758353 by using the basic construction shown in figure 2. For this part we used the repressor RcnR under control of a constitutive promoter ( BBa_K1758350 ) and the nickel specific promoter PrcnA with a 5’UTR in front of sfGFP ( BBa_K1758352 ) as reporter protein.

We tested our nickel sensor with sfGFP as reporter gene, to test the functionality of the system. Moreover we tested different concentrations. The kinetic of our sensors response to different nickel concentrations is shown in figure 3. The first five hours show a strong decrease in fluorescence. After that there is a slight increase in fluorescence. Starting levels of fluorescence are not reached. For better visualization the kinetics of figure 3 are represented as bars in figure 4. A fluorescence level difference for 60 min, 150 min and 650 min is represented.
The data for our nickel sensor show a trend that differs for that of the other sensors. There is no indication for a working sensor in vivo (Figure 3 and 4). There is a fluorescence signal, but it decreases in the first five hours. After reaching a minimum the fluorescence increases slowly. Additionally, there is no difference in fluorescence as response to various nickel concentration. Nickel could influence the cells and thereby caused a precipitation, which could result in decrease of fluorescence. With this sensor no production of sfGFP via fluorescence level change could be detected. Therefore, this sensor is not suitable for our approach. Due to this observation, no in vitro data using CFPS were taken.
To summarize
With this sensor no production of sfGFP via fluorescence level change could be detected. Therefore, this sensor is not suitable for our approach. Consequently, no in vitro tests were performed. To create a working sensor based on this concept further optimization is needed.
To summarize all
We have characterized heavy metal sensors for arsenic, chromium, copper, lead, mercury and nickel. The results for our nickel characterization indicated that the constructed nickel sensor is not suitable for our test strip. The sensors for lead and chromium showed great potential, as they showed responses to chromium or lead, but require further optimization. Copper, our new heavy metal sensor, worked as expected and was able to detect different copper concentrations. The already well-characterized sensors for arsenic and mercury were tested as well. While the arsenic sensor worked well in vivo, it requires some omptimization for the use in vitro. Mercury showed that a fully optimized sensor works very well in our in vitro system and has the potential to detect even lower concentrations than in vivo.












