Team:UChicago/Notebook
Notebook
Document the dates you worked on your project.
What should this page have?
- Chronological notes of what your team is doing.
- Brief descriptions of daily important events.
- Pictures of your progress.
- Mention who participated in what task.
Inspiration
You can see what others teams have done to organize their notes:
GeneHackers Summer 2015 Journal
Original File:UChicagoLabNotebook.pdfWeek 1:
Goals- take inventory, design constructs and primers, test competent cells
6/15/15
Worked on primer design quiz questions. Discussed shift of project direction with Justin based on recently published paper (Chen, 2015): http://advances.sciencemag.org/content/1/5/e1500358.full
Took inventory, created excel sheet online under Protocols folder =E2=80=9CGenehackers 2015 Inventory=E2=80=9D
Downloaded SnapGene Viewer to work on plasmid constructs.
6/16/15
New idea for output system -create one construct with SasA/RpaA as activator of output molecule, another with LabA/RpaA as inhibitor (negative feedback loop) of output molecule. Based on article (Taniguchi, 2010). Started design on constructs, possibly 5 in total:
- Read-out activator (KaiCEE-RFP, RpaA, SasA)
- Read-out inhibitor (KaiCEA-RFP, RpaA, CikA)
- Test gene (kaibc promoter, GFP)
- Fusion SasA
- Fusion KaiC-P
Last two based heavily on paper (Chen, 2015)
Met with Jennifer Moran -need to do safety training before autoclaving liquids and other procedures, will accomplish once more people are back.
To Discuss: Is it worth having negative feedback regulator of RpaA/ output molecule?
6/17/15
Decided to use CikA as negative regulator/inhibitor instead of Lab A. CikA better characterized. Reviewed (Gutu. O=E2=80=99Shea, 2013).
6/18/15
Finished design on constructs 1, 2, 3 Deciding on RBS, perhaps need to use high efficiency promoters and lower efficiency RBS. Justin will email kaibc/Kai analog sequences. Decided on meeting 4pm Tuesday.
To Discuss: Specific RBS and promoter strengths on different genes.
Started Testing for Competent Cells
*Used Justin/Rust Lab protocol instead of iGEM/team protocol because did not have cmrR plates.
Labels:
- Plasmid of Interest- PJ006, containing KaiABC under kaiA, and kaibc promoters, Spec resistance, Conc=3D 100ng/uL
Steps
- Remove cells from freezer, incubate on ice
- Add 1 uL DNA (100ng/uL) into competent cell tube
- Incubate tube on ice for 30 mins
- Incubate tube 42C water bath for 1 min heat shock
- Incubate tube ice 5 mins
- Rescue cells by pipetting 900 uL LB into tube (use sterile flame)
*Used LB from MJR Lab, will have to make LB tomorrow
6/19/15
Checked on Transformed Plates
Good growth on both sections.
Transformation efficiency
0.10 Section
=3D (293 cfus) / ( ((1 uL x 100 ng/uL)/1000 uL soln)(20/200 uL plated))
=3D 293 cfus/0.01 ng DNA plated =3D 2.93 x 10^4 transformants/ng
To Discuss: How to improve Transformation Efficiency.
Made 500 mL LB Solution
Made CM plates
Temperature of Freezer=3D 6 C, Chloramphenicol storage temperature=3D 2-8 C
125 uL of 50mg/ml cm used for every 250 uL plate soln made
Poured plates
Need to label once plates set overnight
Updated restock list, will go tomorrow to buy new supplies (check Inventory)
6/20/15
Stock room closed :(, Will order things on Monday
Placed cmR plates in left cold room, bottom right corner of room behind some Rust plates
Week 2:
Goals- Finalize construct design, develop Competent Cells
6/22/15
Worked on Week 1 Presentation
Streaked 1 Tube of Comp 2014 Cells on LB Only Plate for Competent E.coli procedure -protocol under master list titled =E2=80=9CCompetent E.Coli 6/21/15=E2=80=9D
Started Mini-prep to extract Kai proteins. Inoculated 2 colonies, 1 each in 2mL LB + Spec medium.
6/23/15
Cultured 5 colonies from LB only plate into 10 mL LB
Week 1 meeting today
Meeting Notes:
=E2=86=92 Discussed project direction and construct design. Need to add terminator after RpaA. Need to decide whether or not fusion protein construct worth it. Given that RpaA might have some basal phosphorylation level, induced CikA should present some results. How the constructs are set up now, ideal tests can only be conducted with all three constructs. Don=E2=80=99t need to perhaps mutagenize cut sites as these plasmids will not be final bio-brick. Final biobrick would possibly only have CikA, SasA. Overall consensus is that CikA worth exploring. Need to develop primers ASAP.
=E2=86=92 Need to develop more work on biosynthesis pathway and decide which molecule want to consider as well as what is focus of experiment. Want KaiABC as submitted biobrick so perhaps focusing on biosynthesis pathway is a bit ambitious. Need to consider perhaps alternative, simpler molecule.
=E2=86=92 Will likely assemble using Gibson, order this free kit from RPI.
Contact Danny for competent cells and Justin for Gibson primers
6/24/15
Worked on designing primers. Contacted Danny for competent cell procedure, will complete on Friday. Will conduct both CaCl2 and RbCl2 procedures. Already have streaked LB only plate for competent cells in cold room.
6/25/15
Finished designing primers, will check with Kevin tomorrow. Inoculated two 5mL LB broths with 1-3 colonies each. NEB Gibson Assembly Kit with competent cells arrived!
6/26/15
Carried out competent cell procedure.
https://drive.google.com/open?id=3D0B7wkycR1BRlmWHQ5UGNOaW0xX25ndmE1aUxPU01uclJCVmNV FIX THIS LINK
Used both Rust Lab and RbCl2 as a comparison.
Week 3:
Goals- Test efficiency of competent cells, start as much cloning as possible
6/29/15
Conducted Transformation of CaCl2 and RbCl2.
Spec on LB negative control culture overnight =3D-0.019 A (no growth at all)
=3D (52 cfus) / ( ((1 uL x 50 pg/uL x 1ng/1000pg)/1000uL soln))*((180/200 uL plated))
=3D 52 cfus/(4.5 x 10^-5) ng DNA plated =3D1.15 x 10^6 transformants/ng (Rust)
=3D52 cfus) / ( ((1 uL x 50 pg/uL x 1ng/1000pg)/1000uL soln))*((180/200 uL plated))
=3D 18 cfus/(4.5 x 10^-5) ng DNA plated =3D4.00 x 10^5 transformants/ng (RbCl2)
6/30/15
Primers for first constructs arrived, however at team meeting discussed how plasmids need to be re-designed to effectively compare SasA and CikA. Also CikA will need KaiB, and likely to add RpaB to be consistent with Chen et al. Constructs for Read-Out system were revamped, and constructs for Oscillation system were designed as well.
Week 4:
Goals- Order final gBlocks and primers. Standardize and develop specific, in depth protocols. Practice Western Blots, start writing project report.
7/20/15
gBlocks for Oscillation and Read-Out systems were modified. See Dropbox for final edits and modifications.
7/21/15
gBlocks for Oscillation and Read-Out systems were finally ordered. Primers were designed.
7/22/15
Primers ordered. Reached out to grad advisers for Western Blotting techniques. Researched Gibson Assembly and Western Blot protocols. Will need to research GFP Protocols.
GFP Protocols
http://advances.sciencemag.org/content/advances/1/5/e1500358.full.pdf
7/23
Materials for practice western blot acquired. Gibson Assembly protocol drafted.
7/24
Started western blot. See Rust Lab protocol. Slight changes include 7.5% gel used, cassette assembled not submerged in buffer. Primers diluted and placed in -20 fridge.
7/27
Primary and secondary antibody staining accomplished. Experiments more clearly laid out. Need to start considering plan for pRha inducible promoter and how to alter stoichiometry.
Week 5:
Goals- Finalize and outline protocols, generate explanations for plasmids and background info for wiki and presentation, order materials, gblock assembly
7/28
Western blot procedures expanded. See Aaron=E2=80=99s email about compatible backbones. This week discussed assays -will need to western blot for KaiA before investigating oscillatory system in order to characterize input L-Rhamnose to output Kai A production. Dilutions will occur on log scale first for L-Rhamnose.
7/29
Finished specific protocols -need to ask White lab for sonicator?. Looked up compatibility of backbones. pSB1,3,4 have pMB1 (copy number 100-300/cell), p15A (low-medium 10-12 copy), pSC101 (~5 copies/cell). Will need to construct primers for kaibc/GFP onto the SasA+CikA/SasA plasmids.
7/30/15
Materials reviewed and listed. Meeting with Barry to talk about iGEM as a class. Went over protocols and methods. Allocated who is ordering what.
7/31/15
Gibson Assembly
5 uL of Gibson HiFi Master mix was used in each assembly reaction to minimize amount reagent used. Amount of blocks used, dependent on bps of each block relative to each other. Each gblock diluted in 20 uL of dH2O. Used standardized amount 50 ng of largest block in each assembly. Assembled on ice. Incubated on 50oC heatblock for 1 hour.
PCR
Assembled 5.5 times of 1X Master Mix (not on ice). Dilute 100 uM (100X) primers to 10X primers. 2 uL of primer and 18 uL of dH2O. Aliquoted 49 uL of Master Mix with 1 uL from Gibson Assembly Mix.
Recipe Phusion Master Mix:
o Phusion 5X GC Buffer =3D 10uL x 5.5 =3D 55 uL
o dNTPs 10 mM =3D 1 x 5.5 =3D5.5 uL
o F Primer MC003 10 uM =3D 2.5 x 5.5 =3D13.75 uL
o R Primer MC004 10 uM =3D 2.5 x 5.5=3D 13.75 uL
o Phusion DNAP =3D 0.5 x 5.5 =3D2.75 uL
o H2O =3D 32.5 x 178.75 uL
o DMSO =3D 1.5 x 5.5 =3D8.25 uL
*Should have added only 170.5 uL (31 x 5.5) =E2=80=93Mix slightly more dilute
Thermocycler Settings: 30 cycles, 98o for 30s, 98o for 10s, 65o for 30s, 72o for 1 min, 72o 7 min, Hold 4o
Week 6:
Goals- gblock assembly and Transformation
8/3/15
Decided on backbones:
Oscillator MC001 =E2=80=93 Cmr (standard igem backbone for submitted biobrick)
Readout SasA MC002 =E2=80=93Amp (theoretically want to use with MC001 if successful) =C3=A0 need to add GFP + kai bc
Readout SasA/CikA MC003 =E2=80=93Amp (=E2=80=9C =E2=80=9C) =C3=A0 need to add GFP + kaibc
KaiC Variants MC004-7- Cmr (would never use with MC001)
Agarose Gel Casting
Made 50 mL of 1% Agarose gel. General procedure: added agarose and 1X TAE into ER flask, microwaved until boil, cool under water, poured into tray, added 0.75uL EtBr for visualizing, inserted combs, cool in cold room for 15-20 mins.
Gel Electrophoresis
Loaded 10uL of 6X loading dye into 50uL samples. Loaded 10 uL of 1 kB Plus DNA Ladder from Invitrogen. Seems to be issue w/amount of sample loaded =E2=80=93only 15-29 uL available. Run under 120V for 30 mins. Could be issue with evaporation in thermocycler. Gel too big for tray. Yields of products exist, however seems low. Issue with MC001 =E2=80=93no clear product visible. PCR should be redone.
Image:
Gel Extraction
Gel Weights:
MC001 =E2=80=93N/A
MC004 -0.1536 g
MC005 -0.2048 g
MC006 -0.0965 g
MC007 -0.3962 g
*1mg =3D 1uL
Added 3x uL QG buffer to volume of gel and 1X uL of isopropanol to volume of gel.
PCR
To redo PCR for MC001,4,5,6,7, 1X Master Mix for 15 reactions made. Two reactions for each construct.
PCR Master Mix Recipe-
o Phusion 5X GC Buffer =3D 150 uL
o dNTPs 10 mM =3D 15 uL
o Phusion DNAP =3D 7.5 uL
o H2O =3D 465 uL
o DMSO =3D 22.5 uL
Added 2.5uL of F and R Primers, 44 uL of Master Mix, and 1uL of DNA for each sample. Thermocycler settings- 98o 2 min, 98o 15 s, 69o 30 s, 72o 1 min, 72o 10 min, 4o hold.
PCR Master mix also used for amplifying linear Cmr backbones (diluted in 10uL, used 1 uL of sample for Phusion PCR).
8/4/15
Gel Electrophoresis
Made 100 mL of 1% Agarose gel. 10 uL, 1kB plus Ladder loaded. 35uL MC001, 20uL MC001, 34 uL MC004, 34 uL MC004, 33 uL of MC006,7,8 and Cmr backbones. Products from MC004,5,6,7 and Cmr Linearized backbones extracted using gel punches (borrowed from Rust Lab, need to order more to return). MC001 still not very good yield. Next step to purify MC004-7 and linearized backbones, redo Gibson and PCR of MC001 using gradient thermocycler.
Image:
Gibson Assembly
5 uL of Gibson HiFi Master mix, 1 uL PMC001_b1, 0.5965 uL PMC001_b2, 3.4305 uL H2O, heatblock for 1 hour 50oC.
PCR
Master mix of 70 uL created (calculate ratio of 1X x 7/5)
o Phusion 5X HC Buffer =3D 14 uL
o dNTPs 10 mM =3D 1.4 uL
o Phusion DNAP =3D 0.7 uL
o H2O =3D 43.4 uL
o DMSO =3D 2.1 uL
o DNA =3D1.4 uL
o F Primer MC003=3D 3.5 uL
o R Primer MC004 =3D3.5 uL
10uL Master mix aliquoted into 7 samples.
Thermocycler settings- 98o 2 min, 98o 15 s, 60o, 62o, 64o, 66o, 68o, 70o, 72o 30 s, 72o 1 min, 72o 10 min, 4o hold.
Actual Anneal temperatures- 60.0o, 62.0o, 63.3o, 66.6o, 68.2o, 69.7o, 72.0o
T=3D66.0o, G=3D6.0o for 30 cycles
8/5/15
Gel Electrophoresis
Made 90 mL of 1% Agarose gel. Loaded 10uL of 7 samples(+loading dye). Run for 120V, 30 mins. EtBr cloud on gel seen, only ladder shows visible bands. No other bands visible. Likely error with PCR and addition of EtBr.
Image:
Gel Extraction
Used Promega spin columns/buffer to concentrate in 15 uL of DNA
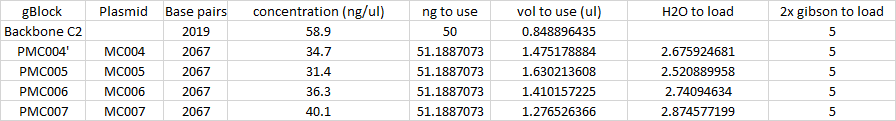
Purity Yields using nanodrop - C1 (Cam Backbone) -
C2 (Cam Backbone) -58.9 ng/uL
4 - 34.7
4=E2=80=99 -
5 - 31.5
6 - 36.3
7 - 40.1
PCR
To redo PCR for Gibson products of MC001, 75 uL of 1X PCR Master Mix
PCR Master Mix Recipe-
o Phusion 5X HF Buffer =3D 15 uL
o dNTPs 10 mM =3D 15 uL
o Phusion DNAP =3D .75 uL
o H2O =3D 46.5 uL
o DMSO =3D 2.25 uL
o DNA (products from Gibson 8/4)=3D 1.5uL
o F Primer MC003=3D 3.75uL
o R Primer MC004 =3D3.75uL
10 uL of Master Mix aliquoted into each sample tube.
To PCR PMC001_b1 for confirmation of block and analysis of primers
50 uL of 1XPCR Master Mix Recipe-
o Phusion 5X HF Buffer =3D 10 uL
o dNTPs 10 mM =3D 1 uL
o Phusion DNAP =3D .5 uL
o H2O=3D 31 uL
o DMSO =3D 1.5 uL
o DNA (pMC001_b1)=3D 1 uL
o F Primer MC005=3D 2.5uL
o R Primer MC006 =3D2.5uL
Added 2.5uL of F and R Primers, 44 uL of Master Mix, and 1uL of DNA for each sample..
Thermocycler settings- 98o 2 min, 98o 15 s, 60o, 62o, 64o, 66o, 68o, 70o, 72o 30 s, 72o 1 min, 72o 10 min, 4o hold.
Actual Anneal temperatures- 60.0o, 62.0o, 63.3o, 66.6o, 68.2o, 69.7o, 72.0o
T=3D66.0o, G=3D6.0o for 30 cycles
8/6/15
Gel Electrophoresis
Made 100 mL of 1% Agarose gel. Loaded 10uL of 7 samples(+loading dye) and 1 50uL sample (divided into two wells, 42uL in one well 18 in the other). Run for 120V, 30 mins. No product clear enough to extract. Imaging gel shows faint products under 68,70, and 72o. Could mean issue with primers. Strangely, no product of right gBlock size seen. Again could be primers.
Image:
Gibson Assembly:
Assembled purified biobricks MC004, MC005, MC006, MC007 into Cam backbone. Used following recipe based on bps of insert and backbone.
Transformation of Assembled products:
DNA straight from Gibson Assembly Reaction was transformed into competent cells. RbCl2 competent cells used. Efficiency of cells: 4.00 x 10^5 transformants/ng (RbCl2)
- Remove cells from freezer, incubate tubes on ice
- Add 1 uL DNA (50 pg//uL) into competent cell tube
- Incubate tube on ice for 30 mins
- Incubate tube 42C water bath for 1 min heat shock
- Incubate tube ice 5 mins
- Rescue cells by pipetting 900 uL LB into tube (use sterile flame)
- Incubate tube in shaker at 37C/1100 RPM for 1 hour
- Heat Cam plate in incubator as cold plate reduces efficiency,complete while cells shaking
- Collect pellet, spin 3000 rcf/gs for 3 mins
- Decant 800 uL of supernatant
- Use glass beads (5-6) per section (use sterile flame)
- Mix pellet, pipetted 200 uL in total
- Shake with beads and remove
- Incubate plate overnight 37C
*Negative control w/no transformed DNA resulted in 0 colonies. Negative control set up on 8/7.
PCR
PCR of 8/3 Gibson PCR, 8/4 Gibson PCR conducted for further amplification. Block 4.1 PCR as positive control. Block 1.1 and 1.2 PCR run to increase DNA amount in hopes of Gibson from amplified blocks. 50 uL of sample for each PCR (5 samples in total).
Added Ingredients to individual Samples
o Phusion 5X HC Buffer =3D 10 uL
o dNTPs 10 mM =3D 1 uL
o Phusion DNAP =3D 0.5 uL
o H2O =3D 31.0 uL
o DMSO =3D 1.5 uL
o DNA =3D1.0 uL
o F Primer =3D 2.5 uL
o R Primer =3D2.5 uL
10uL Master mix aliquoted into 7 samples.
Thermocycler settings- 98o 2 min, 98o 15 s, 70o, 30 s, 72o 1 min, 72o 10 min, 4o hold.
Primers for each sample
Gibson 8/3 -MC003/MC004
Gibson 8/4 -MC003/MC004
pMC001_b1 -MC005/MC006
pMC001_b2 -MC007/MC008
pMC004_b1- MC019/MC020
*Upon further examination, should have used MC003 for pMC004_b1
8/7/15
Gel Electrophoresis
1% Agarose gel run of PCR products from 8/6. Could not see products under blue light. Under UV light, products seemed more specific. Still not as strong as in previous gels. Perhaps need to troubleshoot PCR better.
Image:
Transformation Results
Used iPhone application =E2=80=9CColonyCount=E2=80=9D to assist in counting plates.
Plate Numbers are indicated on lower left of the pictures.
Average is 321 colonies.
[[File:UChicagoPlate1_1.jpg]] [[File:UChicagoPlate1_2.jpg]] [[File:UChicagoPlate1_3.jpg]] [[File:UChicagoPlate1_4.jpg]]Transformation Efficiency
Used 1ul of 50pg/ul of DNA
4: 287 / 5*10^-5 =3D 5.74*10^6 cfu/ug
5: 328 / 5*10^-5 =3D 6.56*10^6 cfu/ug
6: 414 / 5*10^-5 =3D 8.28*10^6 cfu/ug
7: 254 / 5*10^-5 =3D 5.08*10^6 cfu/ug
Primers
Designed Sequencing primers as well as new primers for pMC001. Primers made specifically for oscillator plasmid -overhangs incorporated to make primers longer and more specific.
PCR
Prepared PCR of products seen on gel in morning (G1, G2, 001b1, 001b2, 004b1, used 1 uL of leftover PCR reaction). Used Q5 High Fidelity polymerase, as no Phusion available.
Ingredients:
Q5 High-Fidelity 2X Master Mix- 25 uL
DNA -1 uL
F Primer -2.5 uL
R Primer -2.5 uL
H2O -19 uL
Thermocycler Settings: 98C 30s, 98C 10s, 65C for 30s, 72C for 30s, 72C for 2mins, hold at 4C
30 cycles
Prepared Colony PCR To confirm inserts of pMC004,5,6,7. Used Taq DNA polymerase instead of phusion.
- Pick single colony from plate, place in 50uL of dH2O (acts as DNA template)
- Add following PCR 1X Master Mix for Taq:
5 uL 10X buffer
1 uL dNTPs
1 uL 10 uM primer stock-VF2 and VR
1 uL DNA stock
0.5 uL Taq
41.5 uL H2O
Thermocycler Settings: 95C 2mins, 95C 15s, 55C 15s, 68C 45s (30 cycles), 68C 10m, hold 4C
-> Extend to 1min per kb (look up on product sheet)
8/9/15
Gel Electrophoresis-
Ran 1% Agarose gel 120V, 30 mins. Ran both Colony PCR and Q5 PCR. 5uL each sample loaded. Different ladder used (Quick Load Purple 2-Log from NEB. Same amount of EtBr (0.75 uL) used.
8/10/15
Gel Electrophoresis-
Gel repeated, this time using leftover 45uL of sample. 1kB Plus invitrogen ladder used.
Innoculation-
Due to failure of Colony PCR, colonies were inoculated and incubated. Will conduct direct miniprep on 8/11 and sequence to sequence. This should help in determining if there is a problem with primers/insert or with the PCR.
Gibson Assembly-
Gibson assembly of BH001_b1 and Cam backbone conducted.
PCR-
Set up PCR for biobricks MC002, and MC003 and BH001 (confirmation).
11X PCR Master Mix
PCR Master Mix Recipe-
o Phusion 5X HF Buffer =3D 110 uL
o dNTPs 10 mM =3D 11 uL
o Phusion DNAP =3D 5.5 uL
o H2O =3D 341 uL
o DMSO =3D 16.5 uL
44uL of Master mix, 2.5 uL of F primer, 2.5 uL of R Primer and 1 uL of template DNA used.
DNA Template
Primers
Info
Gel to Run
MC002_b1
MC029, MC010
1815 bps
Clone out b1 to give right initial sequence for kaibc/GFP/Term inserts
1% Agarose
MC003_b1
MC029, MC014
1815 bps
Clone out b1 to give right initial sequence for kaibc/GFP/Term inserts
1% Agarose
MC008_b1
MC003, MC028
1294 bps
Clone out kaibc promoter/GFP
1% Agarose
Biobrick B0015 Terminator
MC027, MC030
129 bps
Clone out terminator
2% Agarose
BH001/Cam Gibson
MC003, MC004
765 bps
*should not have done
? Was to clone out insert, should have transformed as is.
1% Agarose
Thermocycler settings- 98C 2min, 98C 15s, 67C 30s, 72C 1.5 mins, 72C 10min, 4 hold.
8/11/15
Gel Electrophoresis-
2% gel for Terminator cloning sample and 1% gel for other PCR samples (see table) run. 120V for 30 min.
Miniprep
BH protocol
PCR
PCR of Minipreps
(BH protocol)
PCR of MC002/3 blocks
Phusion 7 x of 1X mix
HF Buffer -70 uL
DMSO -10.5 uL
DNTPs -7 uL
H2O -219 uL
Phusion DNAP -3.5 uL
Use 44 uL of master mix w/ 2.5 of F and R primers and 1 uL of template DNA.
DNA Template
Primers
MC002_b1
MC029, MC010
1815 bps product
MC003_b1
MC029, MC014
1815 bps product
MC008_b1
MC003, MC028
1294 bps product
Thermocycler Settings-
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
30 seconds
30 Cycles
98=C2=B0C
65=C2=B0C
72=C2=B0C
10 seconds
30 seconds
1 min (30s x 1.8kb -largest product)
Final Extension
72=C2=B0C
10 min
Hold
4=C2=B0C
Hold
Thermocycler Settings for Mini-Prep PCR-
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
120 seconds
30 Cycles
98=C2=B0C
61.6=C2=B0C
(NEB - DMSO%*0.8)
72=C2=B0C
15 seconds
30 seconds
1 min (30s x 1.8kb -largest product)
Final Extension
72=C2=B0C
5 min
Hold
4=C2=B0C
Hold
8/12/15
PCR of Terminator
2 50 uL Samples
HF Buffer -10 uL
DMSO -1.5 uL
DNTPs -1 uL
H2O -31 uL
Phusion DNAP -0.5 uL
10 uM of MC027 Primer -2.5 uL
10 uM MC030 Primer -2.5 uL
DNA from plate -1 uL
Thermocycler Settings: 1 cycle: 98C for 2 mins, 5 cycles: 98C for 15s, 69C for 30s, 72C for 2 min 30 cycles: 98C for 15s, 72C for 1.5 min, 1 cycle 72C for 10 min, 4C hold.
Gel Electrophoresis-
50 mL 2% gel, 150 mL 1% gel, and 100 mL 1% gel run for Miniprep PCR as well as MC002/3 clone parts PCR. 25 uL of sample loaded for Terminator 2% gel. 19 uL sample loaded for 150mL Miniprep gel. 45 uL of sample loaded for MC002/3 clone parts 1% gel. 1kB plus Invitrogen ladder used. Send samples 41,43,51,52,53,54,61,62,63,64,74 for sequencing.
Images:
Gel Purification-
Extracted correctly sized product fragmnets under blue light: T-189 bps, GFP 1249 bps.
Weights of gels:
GFP 1-63.8 mg
GFP 2-70.64 mg
T 1-34.6 mg
T 2-110.0 mg
Used Machery-Nagel clean up method:
- Add 200uL NTI Binding buffer / 100 mg gel
- Incubate at 50C for 10 mins
- Transfer solution to spin column and collection tube.
- Spin at 11,000g (RCFs) for 30s
- Discard flow through. Add 700 uL of wash buffer NT3 to spin column.
- Spin at 11,000g (RCFs) for 30s
- Discard flow through. Add 700 uL of wash buffer NT3 to spin column.
- Spin at 11,000g (RCFs) for 30s
- Spin at 11,000g (RCFs) for 1 min to dry silica membrane
- Elute DNA with 15 uL of NE elution buffer. Spin at 11,000g (RCFs) for 30s
- Nanodrop (use EB buffer as blank, load 1.5 uL sample)
Purity recorded w/Nanodrop:
T1- 5.1ng/uL 260/280-14.84
T2-23.1ng/uL 260/280-1.92
GFP 1- 41.6ng/uL 260/280-1.94
GFP 2- 33.1ng/uL 260/280-2.03
Plan for MC002_b1, MC003_b1: There is low yield of insert probably due to repeating regions of DNA in blocks 1 of MC002 and MC003. As IDT expressed, there are a lot of low mass products that are more efficiency amplified by primers. Therefore, as Jennifer Moran mentioned, the best course of action would be to insert these two gblocks into a Zero Blunt TOPO PCR Vector and transforming into competent cells to amplify our gblocks. After producing colonies, we can PCR and then screen for colonies with correct product size. We will then miniprep and send these blocks in for sequencing. This will take more time, but will ensure the purity of the insert. After confirming the correct sequence, the DNA from the miniprep/gel extraction? can be used to gibson the GFP/kaibc, the terminator, and the first blocks together.
Mini-Prep Results (Nano-Drop)
Sample
260/280
260/230
ng/ul
41
1.86
2.20
55.1
42
1.84
2.06
35.4
43
1.89
2.10
72.9
44
1.92
1.83
30.7
51
1.94
1.87
46.8
52
1.90
2.00
53.9
53
1.90
2.15
66.6
54
1.93
2.14
58.6
61
1.97
2.06
52.2
62
1.97
2.14
61.1
63
2.00
2.24
52.5
64
1.92
2.12
57.1
71
2.03
2.13
56.9
72
1.88
1.81
50.2
73
1.93
1.83
59.3
74
1.92
1.92
53.3
The highlighted ones are the ones that we choose to sequence.
Sequencing-
The concentration of the DNA templates were too low, so we used 10 ug of each.
The primers were diluted to a 4uM solution from a 100x stock.
(1.4 ul of primer + 33.6 ul of water)
VF2 [1]
MC003 (F) [2]
MC041 (F) [3]
MC023 (F) [4]
MC022 (R) [5]
VR [6]
[41]
[43]
[53]
[54]
[62]
[63]
[71]
[73]
<img src=3D"cid:Image_7.png" />
^Things sent.
Transformation of BH001:
DNA was transformed into competent cells. RbCl2 competent cells used. Efficiency of cells: 4.00 x 10^5 transformants/ng (RbCl2)
8/13/15-
<img src=3D"cid:Image_8.png" />
Transformation Efficiency of BH001-
amt dna used/plate
Plates 1 and 2 were discarded, a colony PCR was done on 6 samples from Plate 3
They were PCRed separately with two different annealing temperatures, 61.6 and 66.
Gibson Assembly
Gibson assembly using 5uL of gibson master mix 2x conducted. Assembled on ice. Incubated for 1 hour 50C heatblock.
PCR-
Prepared 2 50uL 1X PCR samples.
HF Buffer -10 uL
DMSO -1.5 uL
DNTPs -1 uL
H2O -31 uL
Phusion DNAP -0.5 uL
F Primer 10 uM MC003 -2.5 uL
R Primer 10uM MC004 -2.5 uL
DNA (Gibson assembly rxn 8/13) -1uL
Thermocycler Settings-
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
30 seconds
30 Cycles
98=C2=B0C
65=C2=B0C
72=C2=B0C
10 seconds
30 secondsc
1.65 mins (30s x 3.3kb -largest product)
Final Extension
72=C2=B0C
10 min
Hold
4=C2=B0C
Hold
Thermocycler Settings for Mini-Prep PCR- Low Temp
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
120 seconds
30 Cycles
98=C2=B0C
61.6=C2=B0C
(NEB - DMSO%*0.8)
72=C2=B0C
15 seconds
30 seconds
1 min (30s x 1.8kb -largest product)
Final Extension
72=C2=B0C
5 min
Hold
4=C2=B0C
Hold
Thermocycler Settings for Mini-Prep PCR- High Temp
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
120 seconds
30 Cycles
98=C2=B0C
66=C2=B0C
72=C2=B0C
15 seconds
30 seconds
1 min (30s x 1.8kb -largest product)
Final Extension
72=C2=B0C
5 min
Hold
4=C2=B0C
Hold
Gel Electrophoresis-
100 mL of 1% agarose gel run 120V, 30 mins. Samples loaded inclde 45 uL of BH003 biobrick and 20 uL of colony PCRs. 5 uL 1 kB plus Invitrogen ladder loaded. Colony PCRs yielded no results, biobrick BH003 extracted using gel punches (seen in image). First column of BH3 seemed to give very low yield (light band not strong band seen before extraction).
Image-
Gel Extraction-
BH003 biobrick extracted from gel. Machery-Nagel protocol used for gel extraction.
Weight of gel: 85.2 mg
Used Machery-Nagel clean up method:
Nanodrop purity- 14.4 ng/uL, 260/280- 1.55
PCR-
Made 4 1X 50uL samples to PCR ampicillin backbone. Used 1 uL of 25ng/uL amp linearized backbone. Primers MC001 and MC002.
PCR Master Mix Recipe-
o Phusion 5X HF Buffer =3D 50 uL
o dNTPs 10 mM =3D 5 uL
o Phusion DNAP =3D 2.5 uL
o H2O =3D 155 uL
o DMSO =3D 7.5 uL
o DNA (products from Gibson 8/4)=3D 1.0uL
o F Primer MC001=3D 2.5uL
o R Primer MC002 =3D2.55uL
Thermocycler settings:98C 30s, 25 cycles: 98C 10s, 69C 30s, 72C 1.5m, 72.0C 10 min, 4C hold
Gibson Assembly-
Given success of gibson and transformation with BH001, decided to try assemble pMC001 and transform directly. Reaction incubated on heatblock for 1 hour at 50C.
<img src=3D"cid:Image_9.png" />
Gel Electrophoresis-
50 mL of 1% agarose gel made to run amp backbone amplifications. 0.5 uL EtBr used. Gel run 120V for 30 mins. 1 kB Plus Invitrogen ladder used. 45 uL of samples loaded.
Image-
Gel Extraction and Purification-
Ampicillin backbone extracted from gel. Machery-Nagel protocol used for gel extraction.
Weight of gel samples:
A1 -135.8 mg (271.6 uL of binding buffer NTI used)
A2 -201.4mg (402.8 uL of binding buffer NTI used)
Nanodrop concentrations of ampicillin backbone samples
A1 - 32.3 ng/uL, 260/280- 1.84
A2 - 33.4 ng/uL, 260/280- 1.59
Gibson-
Gibson assembly of ampicillin backbone to BH003 insert conducted. Used 5uL of gibson master mix 2x conducted. Assembled on ice. Incubated for 1 hour 50C heatblock.
Transformation of BH003:
DNA was transformed into competent cells. RbCl2 competent cells used. Efficiency of cells: 4.00 x 10^5 transformants/ng (RbCl2)
8/14/15
Transformation-
MC001 and BH003 gave good results from transformation. Colonies were small, but evenly distributed. Colonies could be small because plates incubated late last night.
Colony PCR of BH003/MC001-
Diluted one colony from plates with most growth into 50uL of dH2O to serve as DNA template.
Made 8.5 x of 1X PCR Master Mix:
HF Buffer: 85 uL
DMSO: 12.75 uL
dNTPS: 8.5 uL
H2O: 263.5 uL
DNAP: 4.25 uL
Used 2.5uL of VF2 and VR each and 1 uL of sample DNA.
Thermocycler Settings for Colony
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
10 minutes
30 Cycles
98=C2=B0C
66=C2=B0C
72=C2=B0C
15 seconds
30 seconds
2.5 mins
Final Extension
72=C2=B0C
10 min
Hold
4=C2=B0C
Hold
Sequencing Primers for MC001- MC003,MC031,MC032,MC006,MC033, MC008
8/15/
Gel Electrophoresis
1% Agarose gel run for colony PCR. 1 kB Invitrogen plus ladder used. 50 uL of sample loaded.
8/17
Sequences Submitted
MC001 note submitted on weekend for sequencing. MC001 submitted in morning for sequencing. 4uM primer stock made by diluting in water (1:24 primer:water ratio). 14 uL of miniprepped DNA submitted with 10uL of 4uM primers.
Primers used:
VF2 (1)
VR (2)
MC003 (3)
MC031 (4)
MC032 (5)
MC006 (6)
MC033 (7)
MC008 (8)
Colony PCR
Colony PCR of MC001 set up again as upon closer examination, insert seems too small to be correct.
Diluted one colony from plates with most growth into 50uL of dH2O to serve as DNA template.
Made 9/5x of 1X PCR Master Mix for 10uL samples (used 9.8 uL Mix and 0.2 uL DNA):
HF Buffer: 18.00
DMSO: 2.70 uL
dNTPS: 1.80
H2O: 55.
DNAP: 4.25 uL
Used 4.50 uL of 10X MC003 and MC004 for mix.
Thermocycler Settings for Colony
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
10 minutes
30 Cycles
98=C2=B0C
66=C2=B0C
72=C2=B0C
15 seconds
30 seconds
2.5 mins
Final Extension
72=C2=B0C
10 min
Hold
4=C2=B0C
Hold
Talked with Justin about ordering materials -TOPO kit should come tomorrow.
8/18
Gel Electrophoresis-
0.8% (to separate out larger fragments with more efficiency) agarose gel cast. Gel run on 120V for 30 mins. 1kB plus Invitrogen ladder used. No results from colony PCR, indicating something wrong during PCR. Likely issue with primers? =C2=BE Used instead of VF2 and VR. Have double checked primers are correct -could be issue with insert and primers. Note -issues with evaporation in thermocycler, which is why samples 115,116,117,118,128 could not be loaded.
Image-
Sequencing Results
Sequencing successful for MC004,5,7. MC006 and MC007 samples probably mislabelled as sequencing indicates correct MC006 phosphomimetic is in MC007 sequence and vice versa. Otherwise, MC007,5, and 4 are all correctly sequenced. Point mutation in MC006 (labelled MC007) samples base 244 of Snapgene file. Point mutation seems to be silent (GGC to GGT, translate features lists both as glycine. Four read out KaiC variants seem good to go.
MC001 -still waiting for colony PCR/ specific primers to arrive for assembly
MC002/3 -still waiting for TOPO kit to come for transformation ligation and assembly
MC004/7 -all assembled
BH001 -assembled, sequence verify
BH002 -gblocks not arrived yet
BH003 -assembled, sequence verify
BH004 -gblocks not arrived
Zero-Blunt TOPT Ligation and Transformation
We will be ligating and transforming four samples in order to allow the bacteria to amplify blocks 1 of MC002 and MC003.
- MC002_b1
Ligation
- Combine 2uL product, 1 uL provided salt solution from pTOPO kit, 2uL H20, 1 uL pCR II-Blunt-TOPO vector
- Pipette up and down a few times to mix.
- Incubate 5 min at room temperature.
- No overnight incubation needed! But you can leave it overnight at 4=C2=B0C if you cannot proceed with the transformation right away,
Transformation
- Thaw one vial of Chemically Competent E. coli cells per transformation on ice.
- Add 2=CE=BCl ligation product to the thawed cells. Keep on ice for 30 mins.
- Transfer vials to a 42=C2=B0C water bath for 45 seconds. Immediately return the tubes to ice for 2 min.
- Add 250=CE=BCl room temperature SOC orLB to each vial.
- Incubate for 1 hr at 37=C2=B0C.
- Plate 200=CE=BCl cells on LB + kanamycin plates.
- Incubate overnight at 37=C2=B0C
*The vector confers resistance to kanamycin, NOT ampicillin.
pTOPO kit)
Note: if you do not have a sufficient number of tranformants in 200=CE=BCl, repeat the transformation, do a short spin (20=E2=80=9030 sec) to gently pellet your cells just before plating. Remove 100=CE=BCl of the supernatant, resuspend the cells by pipeting up and down.
PCR
Set up PCR for amplifying MC002_b1 and MC003_b1. The products from this amplification (correct size ~1850-1970 bps) will be ligated and transformed using the TOPO zero blunt kit in order to have more specific inserts.
Set up 2 50uL 1X PCR Reactions:
- dNTPS: 1uL
Thermocycler settings for gBlock PCR
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
30 seconds
30 Cycles
98=C2=B0C
66=C2=B0C
72=C2=B0C
10 seconds
30 seconds
1.65 mins
Final Extension
72=C2=B0C
10 min
Hold
4=C2=B0C
Hold
Innoculation
Colonies from MC001 plates were inoculated as colony PCR results were ambiguous. Samples: 115,116,117,118,125,126,127,128.
- Aliquot enough LB for 2mL/sample into a Falcon tube. Pipette 2mL of LB into each culture tube/sample.
- Pipette all of the colony suspension into the culture tube as well.
- Put in antibiotic to act as primary screening for insert -Cam 1uL, Amp 4uL (antibiotics stored in the -20 fridge)
- Place culture tubes into shaker at 37C overnight (~16 hrs)
Gel Electrophoresis
1% agarose gel used to run gel electrophoresis for MC002_b1 and MC003_b1. Gel run under 120V for 30 mins. Gel extraction of estimated product size conducted under blue light. 5uL 1kB Plus Invitrogen ladder loaded. 50uL of samples loaded.
Image
8/19/15
Transformation Results
Bacterial lawns seen on all plates. Perhaps issue with plates or competent cells are naturally resistant to kanamycin? Will try dilutions if cells are very efficient as cells could also be very conducive to transformation.
Sequencing Results
Analyzed samples 124 and 121 using Blast and chromatograms. Both samples did not have whole insert -MC031 gave no results for both samples. In both samples sequencing only at end of insert (part of KaiC w/KaiB and suffix) resulted.
Miniprep-
Miniprep inoculations 115,116,117,118,125,126,127,128.
- Centrifuge cells at 12000 rcf for 3 mins to harvest cells. Remove all medium.
- Resuspend cells by adding 250uL of resuspension buffer R3 with RNase A. Mix up and down until homogenous.
- Add 250uL of Lysis buffer (L7) to lyse cells. Mix gently by inverting capped tube.Do not vortex, incubate at room temp for 5 mins.
- Add 350uL of precipitation buffer N4. mix immediately by inverting tube or vigorously shaking. Do not vortex, centrifuge at 20,000 rcfs for 10 mins.
- Load supernatant (750 uL) into Spin column (machery nagel used) and collection tube. Centrifuge column at 12,000 rcfs for 1 min. Discard flow through and place column back in wash tube.
- Add 500 uL of wash buffer W10 with ethanol. Incubate at room temp for 1 min, centrifuge column at 12,000gs for 1 min. Discard flow through. (*Optional Wash step)
- Add 700 uL of wash buffer W9 with ethanol. centrifuge column at 12,000 gs for 1 min. Discard flow through then dry spin for 1 min at 12,000 gs. Discard flow through.
- Place spin column into microfuge tube. Add 50uL of TE Buffer to center of column. Incubate column in room temp for 1 min.
- Centrifuge column at 12,000 rcfs for 2 mins. Discard column. Nanodrop DNA. Store at 4C short term, -20C long term.
Nanodrop results-
115: 91.8 ng/uL, 260/280: 1.90
116: 172.0 ng/uL, 260/280: 1.82
117: 152.0 ng/uL, 260/280: 1.91
118: 120.4 ng/uL, 260/280: 1.81
125: 187.0 ng/uL, 260/280: 1.88
126: 149.2 ng/uL, 260/280: 1.88
127: 211.0 ng/uL, 260/280: 1.87
128: 222.8 ng/uL, 260/280: 1.89
PCR
Set up PCR for both miniprep PCR and PCR of MC002_b1 and MC003_b1 to redo ligation and transformation.
Set up 2 50uL 1X PCR Reactions for MC002_b1 and MC003_b1:
Made 9/5x of 1X PCR Master Mix for 10uL samples (used 9.8 uL Mix and 0.2 uL DNA):
HF Buffer: 18.00
DMSO: 2.70 uL
dNTPS: 1.80
H2O: 55.
DNAP: 4.25 uL
Used 4.50 uL of 10X MC003 and MC004 for mix.
Thermocycler Settings (used 2 thermocyclers, one for 10uL samples and other for 50uL samples)-
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
30 seconds
30 Cycles
98=C2=B0C
67=C2=B0C
72=C2=B0C
10 seconds
30 seconds
1:40 mins
Final Extension
72=C2=B0C
10 min
Hold
4=C2=B0C
Hold
Gel Electrophoresis-
1% agarose gel run at 120V for 30 mins. Loaded 10uL and 45 uL for minipreps and MC002/3 gel extractions respectively. Loaded 5uL of 1kB Plus Invitrogen ladder.
Image-
<img src=3D"cid:Image_10.png" />
8/20
Sequences Submitted
MC001 samples submitted in morning for sequencing. 4uM primer stock made by diluting in water (1:24 primer:water ratio). 10 uL of miniprepped DNA submitted with 10uL of 4uM primers. Samples 115,117, 125, 127, 128 sent.
Primers used:
VF2 (1)
MC003 (2)
MC031 (3)
MC032 (4)
MC006 (5)
MC033 (6)
MC008 (7)
VR (8)
Gibson Assembly
New primers for MC001 arrived. Proceeded with Gibson assesmbly. Incubated mix at 50C for 1 hour.
PCR
Set up PCR for MC002_b1, MC003_b1, MC001_b1, MC001_b2, and MC001 Gibson products. Total 7 reaction (MC002/3 block 1s sampled twice as one will be used for gel extraction then TOPO ligation/transformation, the other will be used to amplify a second time).
Phusion 7.5 x of 1X mix
HF Buffer -75.00 uL
DMSO -11.25 uL
DNTPs -7.50 uL
H2O -232.50 uL
Phusion DNAP -3.75 uL
Add 44uL of PCR Mix to 2.5 of F and R Primer each and 1 uL of DNA
DNA : Primers
MC002_b1 : MC029, MC010
MC003_b1 : MC029, MC014
Gibson rxn : 1b1F, 1b2R
MC001_b1 : 1b1F, 1b1R
MC002_b2: 1b2F, 1b2R
Thermocycler settings:
Thermocycler Settings (used 2 thermocyclers, one for 10uL samples and other for 50uL samples)-
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
30 seconds
30 Cycles
98=C2=B0C
66=C2=B0C
72=C2=B0C
10 seconds
30 seconds
1:30 mins
Final Extension
72=C2=B0C
10 min
Hold
4=C2=B0C
Hold
Gel Electrohporesis-
1% Agarose gel run at 120V for 30 mins. 45 uL of samples loaded. Hard to see bands under blue light. Used UV light for quick clarification of size. No products from MC001 Gibson seen. MC001_b1 and MC001_b2 extracted separately.
8/21
PCR
Due to failure of yesterday=E2=80=99s PCR, gradient PCR was set up in order to determine optimal anneal temperature.
Master mix of 75 uL created (calculate ratio of 1X x 7.5/5)
o Phusion 5X HF Buffer =3D 15 uL
o dNTPs 10 mM =3D 1.5 uL
o Phusion DNAP =3D 0.75 uL
o H2O =3D 46.5 uL
o DMSO =3D 2.25 uL
o DNA =3D1.5 uL
o F Primer MC003=3D 3.75 uL
o R Primer MC004 =3D3.75 uL
10uL Master mix aliquoted into 7 samples.
Thermocycler settings- 98o 2 min, 98o 15 s, 60o, 62o, 64o, 66o, 68o, 70o, 72o 30 s, 72o 1 min, 72o 10 min, 4o hold.
Actual Anneal temperatures- 60.0o, 62.0o, 63.3o, 66.6o, 68.2o, 69.7o, 72.0o
T=3D66.0o, G=3D6.0o for 30 cycles
Gel Purification
MC001_b1 and MC001_b2 extracted yesterday were purified. Used Machery-Nagel protocol.
Weights-
1b1 -13.4 mg
1b2 - 133.4 mg
Nanodrop results-
1b1 - 13.1 ng/uL
1b2 -19.2 ng/ul
Gel Electrophoresis
1% agarose gel run for gradient PCR. 120V for 30 mins. 1kB Invitrogen ladder used. 10uL of sample loaded. Expected product size ~3000 bps. No expected product size appeared on gel. Perhaps indicates issues with Gibson assembly. 24 inoculated colony PCRs also run on separate gel. 3 colonies chosen for inoculation and miniprep: 3,9 and 22.
Image-
<img src=3D"cid:Image_11.png" />
<img src=3D"cid:Image_12.png" />
Gibson-
Gibson of MC001 repeated. Incubated mix at 50C for 1 hour.
PCR-
PCR set up for MC001 blocks and MC001 8/21 Gibson. 6 samples in total. 1X PCR Mix for each sample set up:
DMAP: 0.5 uL
DMSO: 1.5 uL
dNTPS: 1 uL
DNA: 1uL
HF Buffer: 10 uL
H2O: 31 uL
F Primer: 2.5 uL (10uM)
R Primer: 2.5 uL (10uM)
DNA (Primers): MC001_b1 (MC001_b1_F_new, MC001_b1_R_new), MC001_b1 (MC001_b2_F_new, MC001_b2_R_new), MC001 Gibson (MC001_b1_F_new, MC001_b2_R_new)
PCR Conducted one at high anneal temperature (68C) one at low anneal temperature (64C)
Thermocycler Settings (used 2 thermocyclers, one for 10uL samples and other for 50uL samples)-
STEP
TEMP
TIME
Initial Denaturation
98=C2=B0C
30 seconds
30 Cycles
98=C2=B0C
68 or 64=C2=B0C
72=C2=B0C
10 seconds
30 seconds
1:30 mins
Final Extension
72=C2=B0C
10 min
Hold
4=C2=B0C
Hold
Gel Electrophoresis-
1% agarose gel run at 120V for 30 mins. 1kB Plus invitrogen ladder used. 50uL of samples loaded. Results very inconclusive.
Image-
8/24
Miniprep-
Inoculated TOPO MC002_b1 and MC003_b1 samples miniprepped using invitrogen protocol.
Miniprep inoculations 115,116,117,118,125,126,127,128.
- Centrifuge cells at 12000 rcf for 3 mins to harvest cells. Remove all medium.
- Resuspend cells by adding 250uL of resuspension buffer R3 with RNase A. Mix up and down until homogenous.
- Add 250uL of Lysis buffer (L7) to lyse cells. Mix gently by inverting capped tube.Do not vortex, incubate at room temp for 5 mins.
- Add 350uL of precipitation buffer N4. mix immediately by inverting tube or vigorously shaking. Do not vortex, centrifuge at 20,000 rcfs for 10 mins.
- Load supernatant (750 uL) into Spin column (machery nagel used) and collection tube. Centrifuge column at 12,000 rcfs for 1 min. Discard flow through and place column back in wash tube.
- Add 500 uL of wash buffer W10 with ethanol. Incubate at room temp for 1 min, centrifuge column at 12,000gs for 1 min. Discard flow through. (*Optional Wash step)
- Add 700 uL of wash buffer W9 with ethanol. centrifuge column at 12,000 gs for 1 min. Discard flow through then dry spin for 1 min at 12,000 gs. Discard flow through.
- Place spin column into microfuge tube. Add 50uL of TE Buffer to center of column. Incubate column in room temp for 1 min.
- Centrifuge column at 12,000 rcfs for 2 mins. Discard column. Nanodrop DNA. Store at 4C short term, -20C long term.
Nanodrop results-
A21:
A22:
A31:
A32:
B21:
B22:
B31:
B32:
None were sufficient enough to send for sequencing. Likely errors also due to overgrowth of colonies.
PCR
Gradient PCR of only MC001 blocks conducted.
Master mix of 75 uL created (calculate ratio of 1X x 7.5/5)
o Phusion 5X HF Buffer =3D 37.5 uL
o dNTPs 10 mM =3D 3.75 uL
o Phusion DNAP =3D 1.875 uL
o H2O =3D 116.25 uL
o DMSO =3D 5.625 uL
o DNA =3D3.75 uL
o F Primer MC003=3D 9.375 uL
o R Primer MC004 =3D9.375 uL
10uL Master mix aliquoted into 7 samples.
Thermocycler settings- 98o 2 min, 98o 15 s, 60o, 62o, 64o, 66o, 68o, 70o, 72o 30 s, 72o 1 min, 72o 10 min, 4o hold.
Actual Anneal temperatures- 60.0o, 62.0o, 63.3o, 66.6o, 68.2o, 69.7o, 72.0o
T=3D66.0o, G=3D6.0o for 30 cycles
Gel Electrophoresis
1 % agarose gel run for 30 mins at 120V. 25 uL of samples loaded. 1kB plus Invitrogen ladder loaded. Only sufficient product seen for block 2. Indicates issues with block 1.
Image
<img src=3D"cid:Image_13.png" />
8/25
Sequencing Results: Found a sample (127) containing entire MC001 insert!
Innoculated sample 127 and prepared for induction with L-Rhamnose.
Re-plated TOPO reactions for MC002/3 as overgrowth of colonies on previous plates.
Set up inductions however, cultured at 37C instead of 30C. Pay attention to this, this is important, all the protocols have induced L-Rhamnose at 30C
8/26
Start over the inductions, follow the igem protocol file for help. You will need to make more LB. I have already made a few glycerol stocks. You will need to set up a 2mL innoculation in a culture tube as well as a 40mL innoculation in a flask. The 40mL innoculation will be used for inductions. [Finished]
Redo TOPO transformations.
8/27
8/28
8/31
Reviewed work done over past three days. Discussed ways to resolve western blot issue. Seems to be a lot of non-specific binding -we see blots of many different sizes. Blots of antibodied products are very bright relative to ladder. Kevin mentioned degradation could be an issue (would cause lots of different sizes in blots). On closer examination, 0% rhamnose has no (barely any) protein. Updated notebook, e-mailed Justin and Kevin for trouble shooting.
BH- Type up lysing assay and bradford assay -give some specifics about western blot (how much each sample, and calculations)
E-mail sleep people
9/1
Induction Solutions
In order to re-do induction western blots, made new 10% Rhamnose w/v solution by diluting 1 g of Rhamnose in 9 mL of water to bring final volume to 10mL.
Culture
Set up two 40 mL cultures of sample 127 in 250 mL flasks. Added 20uL of Cam in each, 40 mL of LB and 10uL of 2 mL sample 127 overnight culture. Note: 2mL overnight culture has been stored in room temperature for past four days -may also have to start from new glycerol stock. 40mL cultures placed in 37C incubator at 11:50.
Dilutions of Rhamnose Inducer Samples
As discussed in meetings, errors with previous western blot likely to be caused due to overexpression of pRhamnose. iGEM team used low copy number plasmid. Re-made inducer solutions using 1/10 of % from 0.001 - 0.1% in order to test lower gradient. Induction started at 2:30 am when OD600 of 40 mL innoculations was 1.6(?)
9/2
Minimal Media
Ordered M9 minimal media 5X salts on Rust Lab tab. Should take till friday to arrive.
Restriction Digest
Protocol from iGEM.
Digest
- Enzyme Master Mix for Plasmid Backbone (25ul total, for 5 rxns)
- 5 ul NEB Buffer 2 (use CutSmart)
- Digest Plasmid Backbone
- Add 4 ul linearized plasmid backbone (25ng/ul for 100ng total)
Ligation
- Add 2ul of digested plasmid backbone (25 ng)
Restreaked TOPO plate
Transformed new TOPO reactions for MC02/3 and BH002/3
Set up Gibson of BH002/3
9/3
Lysed cells after induction
Bradford Assay
Conducted Western blot
Ran colony PCR of TOPO reactions -only MC003 TOPO worked. BH002/3 Gibsons worked, no TOPO results for MC002, BH002, BH003.
Inoculated negative control.
9/4/15
Set up Rhamnose time course experiment and rhamnose gradient experiment. Both used M9 media and LB media. M9 media arrived.
9/7/15
Miniprepped colony PCR for MC003, BH002, BH003.
9/8/15
Set up bradford of time-course and rhamnose gradient. Ran gel and transfer of assays. Ran negative vector control this time.
9/9
Finished western blot, imaged gel. Blotted only for KaiA and KaiC.
9/10
Set up induction again, be careful of transfer where errors seem to occur. Blot for Kai B from previous assay.
</html>
Like our team Facebook page, Genehackers@UChicago!
Questions? Comments? Send us an email!