Team:Nagahama/Experiments
Contents
Result and Discussion
Confirmation antibacterial activity of each volatile substances derived from plant
First, we examined our working hypothesis to “Flavorator” that the volatile gaseous substances from plants’ origin can show either the antibacterial or bacteriostatic activity in a box like “KOZOKO”. The results clearly showed that all the volatile substances of wasabi(Japanese horse radish), rose, garlic and onion had antibacterial properties. In the literatures, wasabi, rose, garlic and onion have antibacterial volatiles, such as allyl isothiocyanate (wasabi), geraniol (rose),allicin (garlic), and lachrymatory-factor (onion). These antibacterial volatiles are produced after complicated pathways, so for their syntheses, various enzymes are required. Then, we searched metabolic pathways in E. coli, in which antibacterial volatiles can be either end products or intermediates. The search hit the geraniol. In E. coli, a precursor of geraniol, geranyl diphosphate (GPP), is synthesized. If we can successfully operate one enzyme to E. coli, it may synthesize geraniol using geranyl diphosphate as a precursor. In this context, we designed our system for establishing the concept of “Flavorator” to build up a brand-new biosynthetic pathways, in which geraniol is produced in the E. coli. In doing so, we transfered the three types of genes listed below to create the hyper-producer E. coli of geraniol.
We examined our working hypothesis to “Flavorator” that the volatile gaseous substances from plants’ origin can show either the antibacterial or bacteriostatic activity in a box like Kozoko.
Preventive effect of grated garlic
Protocl here
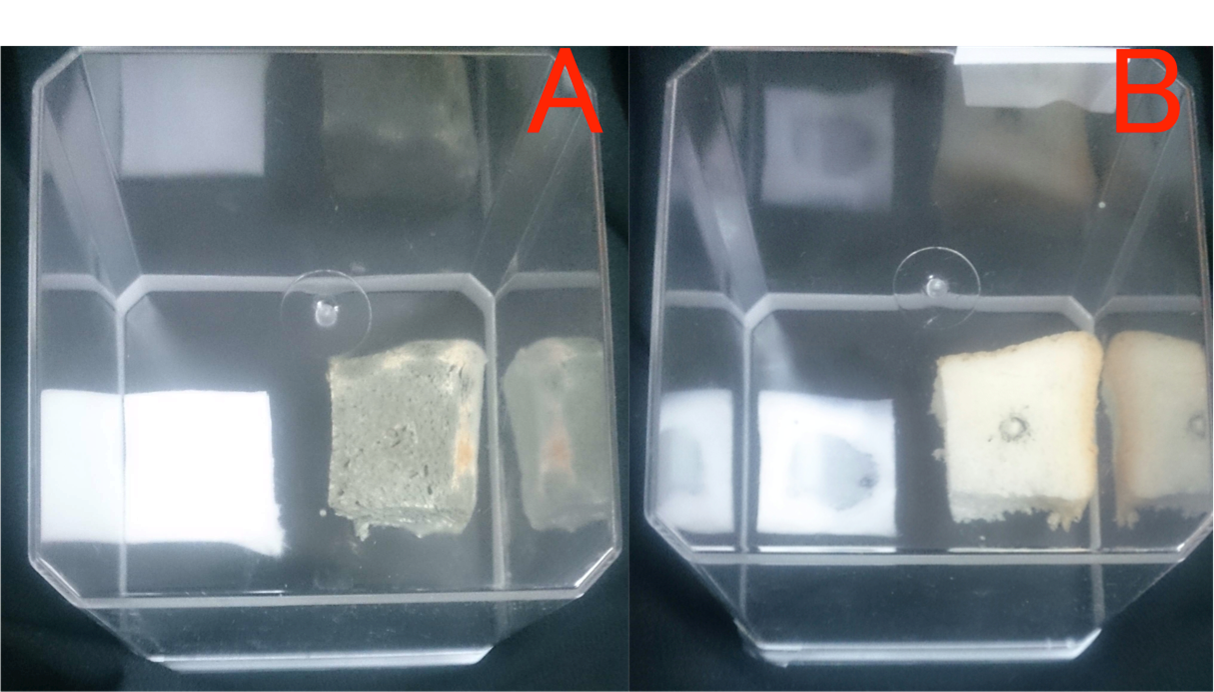
Garlic was known to produce its flavorous substance (alicin) to suppress unwanted microbial growth. We checked the effect of volatile substance, whether it is effective to prervent mean deay or not. The left figure (A) shows that the pink color of the fresh pork meat was not changed. The right one (B) shows that the color of the pork meat was changed from pink to brown. Based on these observations, grated garlc has the preventive(s) to protect the pork's decay, possibly by releasing the antibacterial substances from the garlic.

(A): the grated garlic paste was put in a small box and it was put in a plastic cage with the separation from the the pork meat without direct contact. (B): the above treatment was not done. These pork meats in a plastic cage were left in the box at 18℃ for 2 months. Lef t pork (A) didn’t change the color. Right pork (B) changed the color from pink to brown.
Preventive effect of grated Wasabi root
Protocl here
Grated Wasabi root produced its fragrances to suppress unwanted microbial growth. We spread the wasabi grated on the rice cake. The rice cake didn’t change the color. We found from this result that fragrance of plants have antibacterial volatiles.
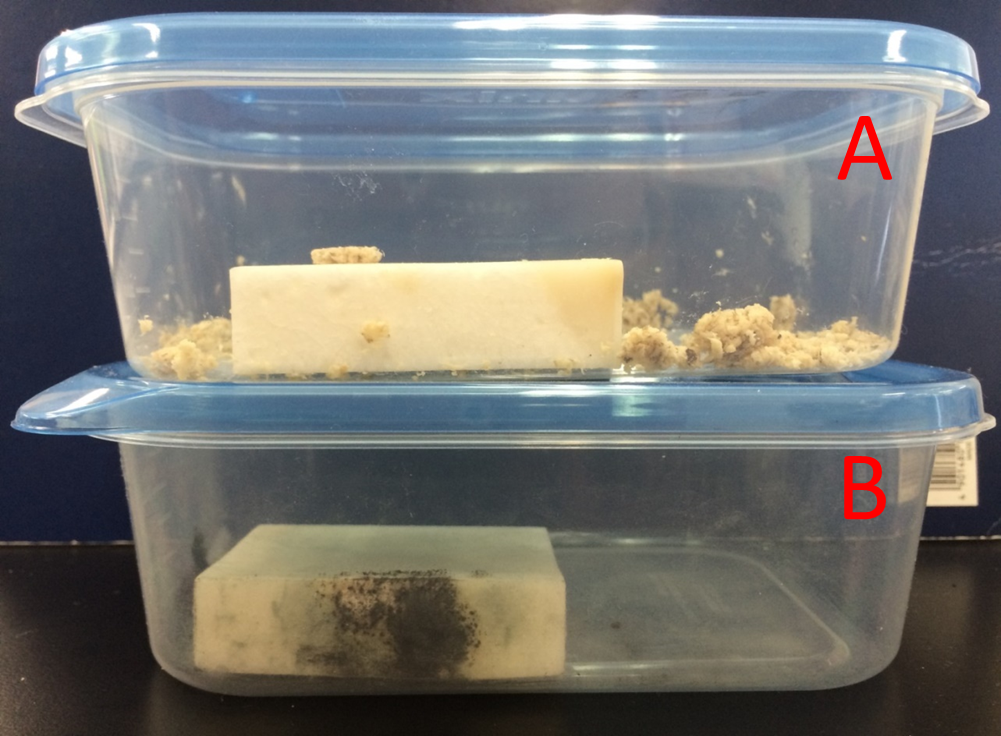
The grated Wasabi root paste was put aside of rice cake in a plastic box without direct contact between rice cake and Wasabi root paste. These boxes were kept at 18℃ for 2 month. The rice cake in figure (A) didn’t change the overall state, while rice cake in figure (B) had mold. (A), plus Wasabi root paste; (B) minus Wasabi root paste.
Confirmation of anti-mold and antibocatrial activities of geraniol and farnesol
Protocl here
Grated roses are known to produce its fragrances to suppress unwanted microbial growth. There are case that the fragrances from plants have antibacterial volatiles.
Increasing in the amount of terpene's precursors
 terpene's precursors device ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1653024 BBa_K1653024])
terpene's precursors device ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1653024 BBa_K1653024])
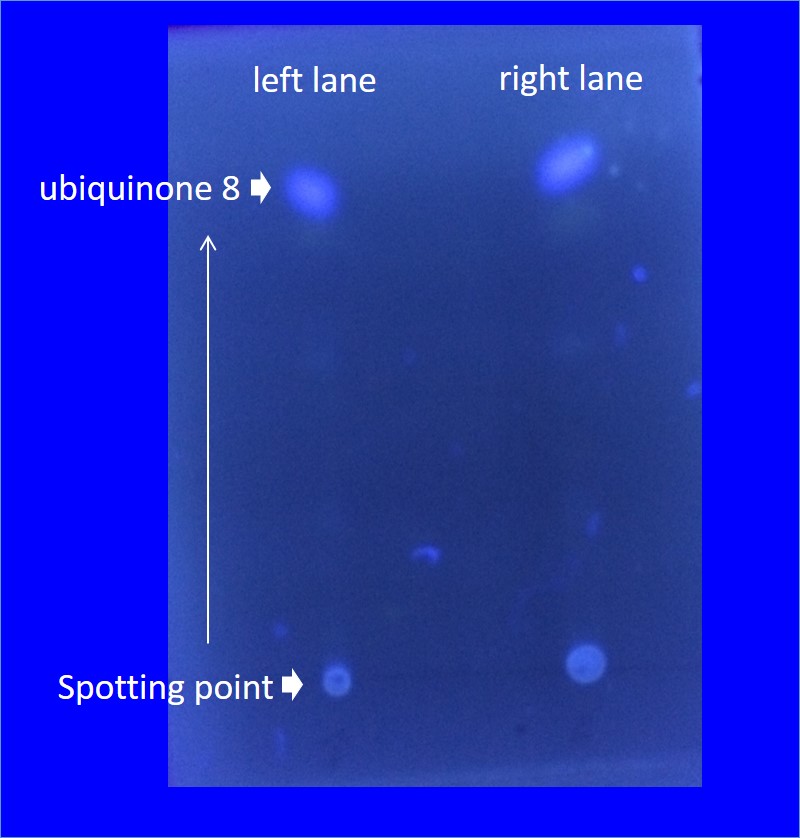
We want to make the E. coli produces farnesol and geraniol which are one of the terpenes. To produce great quantity of terpenes they need many terpene precursor. E. coli produces a small amount of the terpene precursor in MEP pathway. In MEP pathway, there are four enzymes (ispD, ispF, idi, dxs) which are speed limiting enzyme for terpenes precursors produce in E. coli. In order to create a high-yield strains producing IPP and DMAPP, we exogenously engineer to superimpose these genes into E. coli to create strains overproducing IPP and DMAPP in a MEP pathway. To confirm increased production of terpene precursors by Terpene precursor mass-production device. We put attention on ubiquinone. Ubiquinone 8 is made from Farnesyl diphosphate (FPP) which is one of the terpene precursors. quinone is one of the electron carrier present in the cell membrane of prokaryotes. And also they glow when exposed to UV rays. In the measurement of production of quinone it was measured by thin-layer chromatography. (TLC silica gel)
Result:
The figure on the left is analysis of Ubiquinone 8 by thin-layer chromatography. Right lane is JM109 /Terpene precursor mass-production device with IPTG Left lane is JM109/Terpene precursor mass-production device IPTG minus . Both it was 2μl spot.
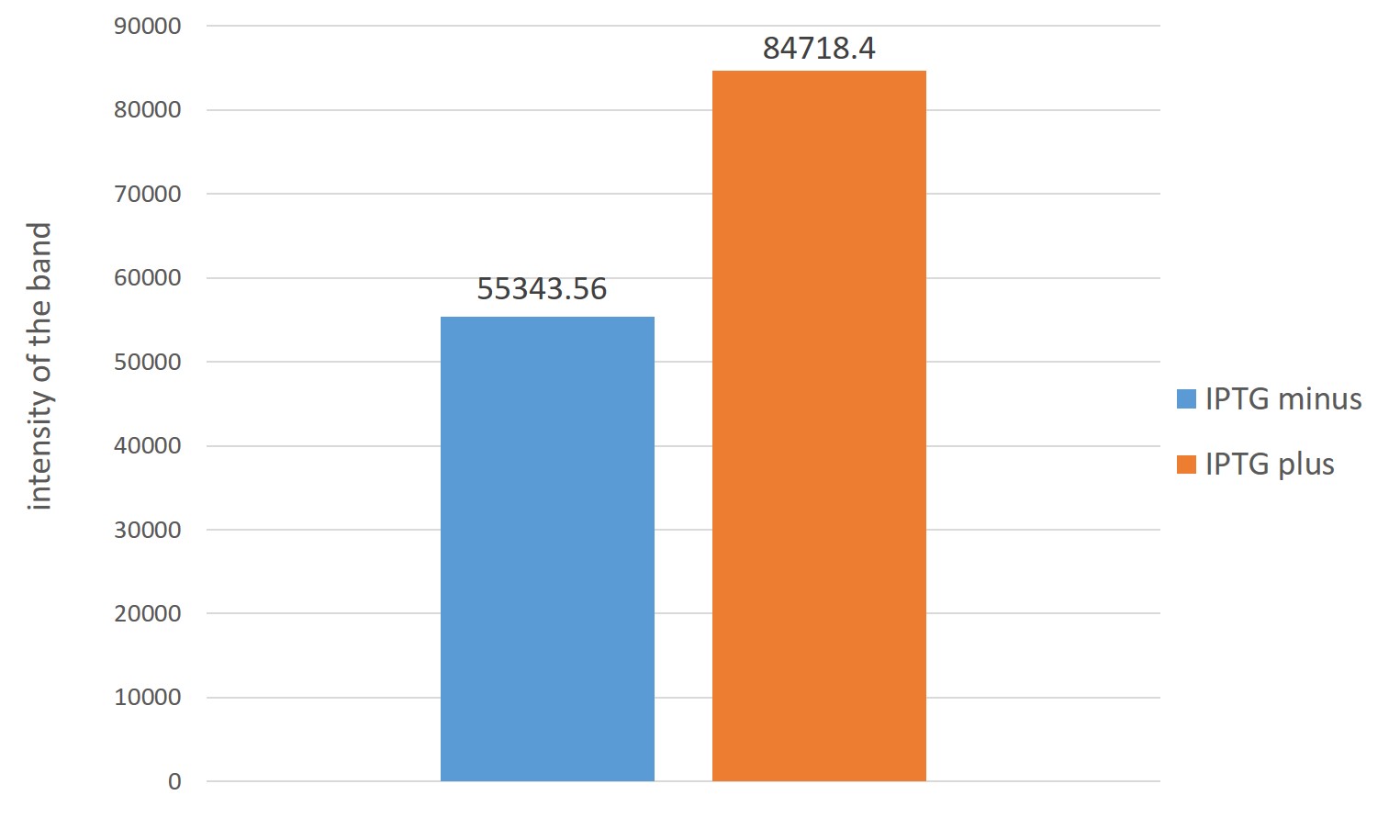
The right of the figure,Estimation of ubiquinone-8 content instead Each intensity of spots indicating the content of Ubiquinone-8
From two figures, those which are overexpressed in reintroduced to E. coli four genes, it is better to have overexpressed were many production of Ubiquinone 8 as compared with those that do not overexpress
Discussion:
From this result, the amount of Ubiquinone 8 of the final material by the increased amount of terpene precursors is increased by re-introducing the four genes are over-expressed in E. coli.
Therefore, it considered could strengthen the MEP pathway.
○protocol here
Produce of Geraniol and Farnesol
Geraniol production
Geraniol production device ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1653025 BBa_K1653027])

Geraniol is generated through GPP hydrolysis by geraniol synthase. A MEP pathway has been shown to synthesize IPP and DMAPP efficiently in E. coli. E. coli engineered with Geraniol production device (BBa_K1653027) showed different smell as compared with the counterpart control (pSB1C3) and the original strain JM109. This result was obtained by the double blind test following the questionnaire survey. And then we tried to detect that the geraniol generated by engineered E. coli by GC and GC-MS. Geraniol was not detected. These results may suggest that E. coli with Geraniol production device produces less than the lowest limit detected by GC and GC-MS. And geraniol synthetase was not detect by CBB stain. And recombinant E. coli LB21 needed 3 days to grow. There were few coloy in LB plate. These result may suggest that E. coli couldn't grow with few graniol, because graniol has very stong antimirobial.
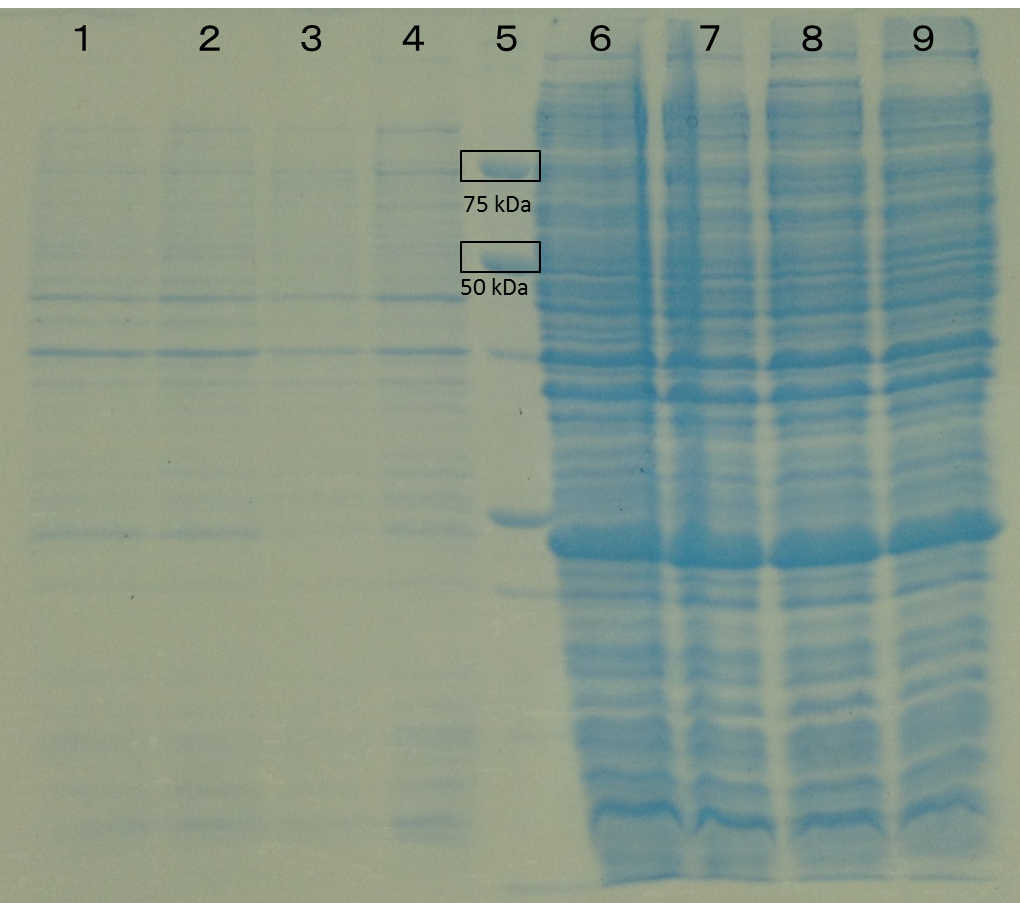
Lane1: E. coli BL21/pET26b Lane2: E. coli BL21/pET26b Lane3: E. coli BL21/pET26b+ObGES Lane4: E. coli BL21/pET26b+ObGES Lane5: Protein Marker(Precision Plus Protein Standards (BIO-RAD)) Lane6: E. coli BL21(Additon of IPTG 1mM) Lane7: E. coli BL21 Lane8: E. coli BL21/pET26b(Additon of IPTG 1mM) Lane9: E. coli BL21/pET26b OD600=0.68 IPTG 1mM ObGES has 1707 bp. It is about 62.59 kDa.

-
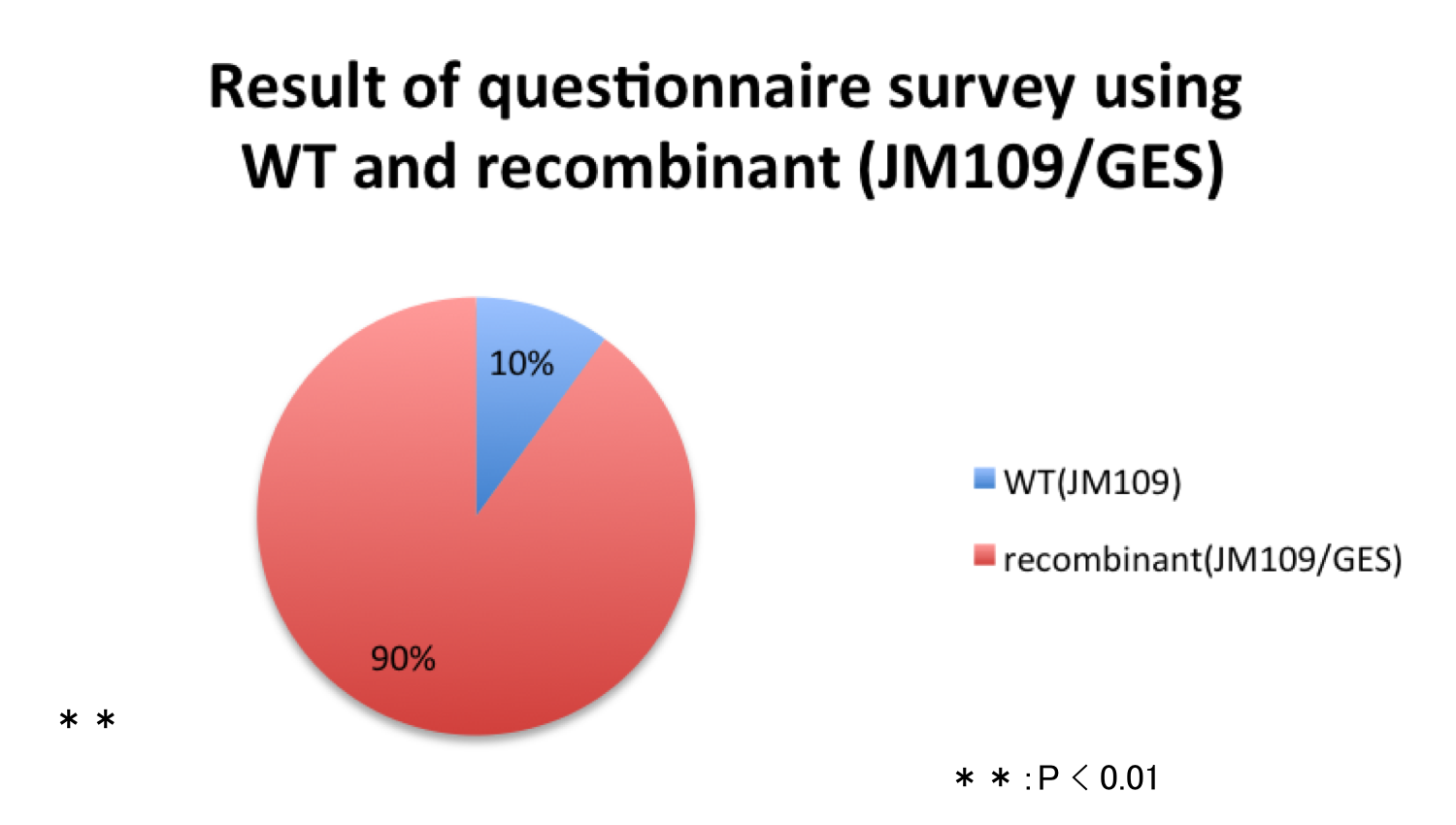 Fig.10 Result of questionnaire survey using WT and recombinant (JM109/GES). Out of 20 persons, two persons (10%) answered the medium A (WT) smelled stronger than the medium B (recombinant (JM109/GES)) and eighteen persons (90%) answered the medium B smelled stronger than the medium A. If we assume that both media smell equally, the probability that the medium A is selected in the questionnaire must be 0.5. From this assumption, p-value of this result was calculated using binomial test. Because the p-value was much smaller than the 5% significance level (0.0004025), the smell of recombinant (JM109/GES) is stronger than that of WT significantly. This result indicate that the recombinant (JM109/GES) synthesize geraniol.
Fig.10 Result of questionnaire survey using WT and recombinant (JM109/GES). Out of 20 persons, two persons (10%) answered the medium A (WT) smelled stronger than the medium B (recombinant (JM109/GES)) and eighteen persons (90%) answered the medium B smelled stronger than the medium A. If we assume that both media smell equally, the probability that the medium A is selected in the questionnaire must be 0.5. From this assumption, p-value of this result was calculated using binomial test. Because the p-value was much smaller than the 5% significance level (0.0004025), the smell of recombinant (JM109/GES) is stronger than that of WT significantly. This result indicate that the recombinant (JM109/GES) synthesize geraniol. -
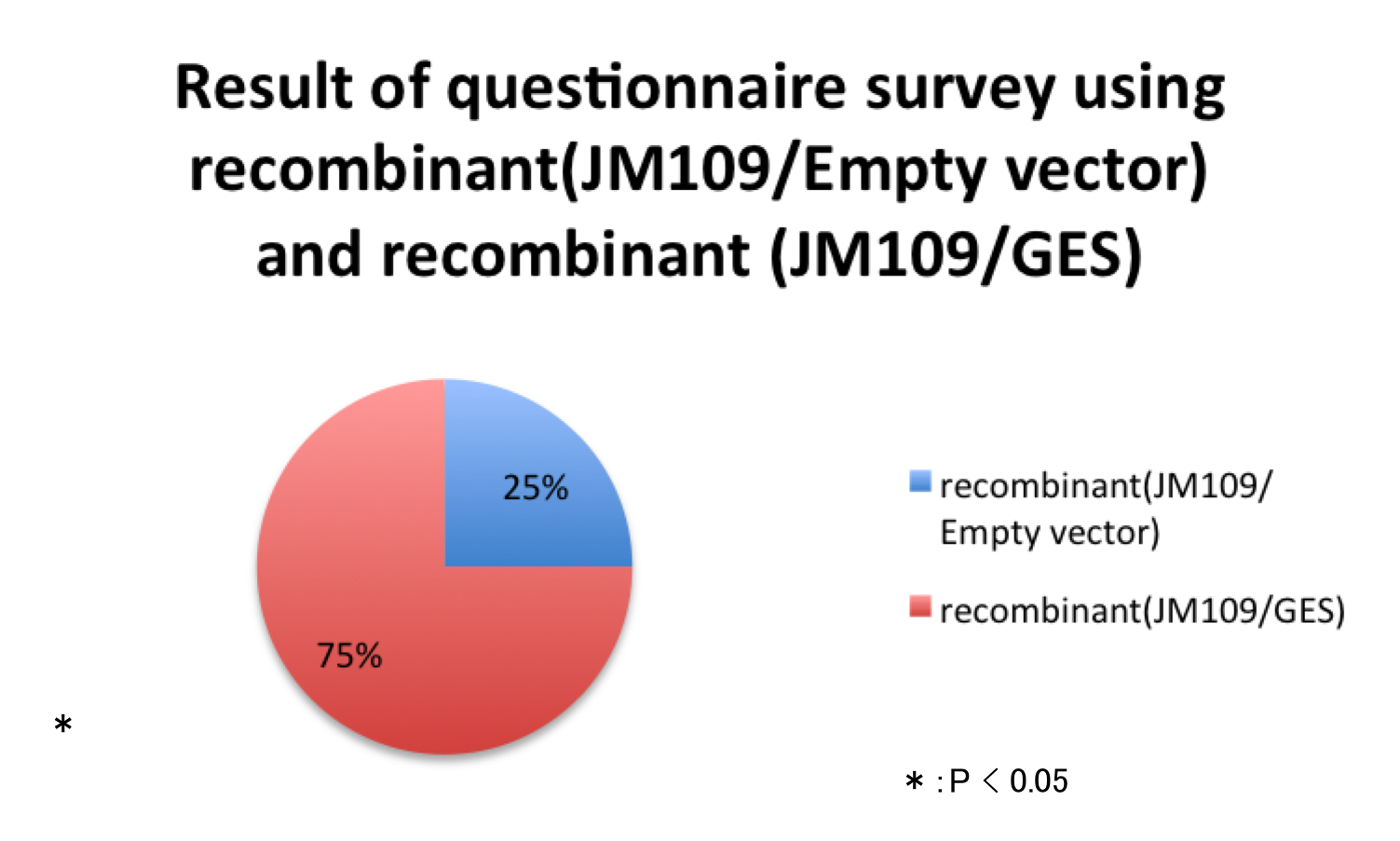 Fig.11 Result of questionnaire survey using recombinant(JM109/Empty vector) and recombinant (JM109/GES). Out of 20 persons, five persons (25%) answered the medium A (recombinant (JM109/GES)) smelled stronger than the medium B (recombinant (JM109/GES)) and fifteen persons (75%) answered the medium B smelled stronger than the medium A. If we assume that both media smell equally, the probability that the medium A is selected in the questionnaire must be 0.5. From this assumption, p-value of this result was calculated using binomial test. Because the p-value was much smaller than the 5% significance level (0.0413900), the smell of recombinant (JM109/GES) is stronger than that of recombinant(JM109/Empty vector) significantly. This result indicate that the recombinant (JM109/GES) synthesize geraniol.
Fig.11 Result of questionnaire survey using recombinant(JM109/Empty vector) and recombinant (JM109/GES). Out of 20 persons, five persons (25%) answered the medium A (recombinant (JM109/GES)) smelled stronger than the medium B (recombinant (JM109/GES)) and fifteen persons (75%) answered the medium B smelled stronger than the medium A. If we assume that both media smell equally, the probability that the medium A is selected in the questionnaire must be 0.5. From this assumption, p-value of this result was calculated using binomial test. Because the p-value was much smaller than the 5% significance level (0.0413900), the smell of recombinant (JM109/GES) is stronger than that of recombinant(JM109/Empty vector) significantly. This result indicate that the recombinant (JM109/GES) synthesize geraniol.
Farnesol production
Farnesol production device ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1653025 BBa_K1653025])
E. coli strain engineered with MEP pathway enzymes, ispDF, idi, and dxs , in combination with the enzyme gens, ispA, produced farnesol (Fig. 11B), which was detected by the Gas chromatography/Mas (Fig. 11A-G), having the same retention time as the farnesol chemical sample (Fig. 11A), while the counterpart control E. coli did not produce farnesol under the same conditions (Fig. 11C). Neither E. coli engineered with MEP pathway enzymes only nor the one engineered ispA only showed any farnesol by the Gas chromatography/Mass (Figs. 11D and E). Farnesol is generated through hydrolysis of farnesyl diphosphate (FPP) by the endogenous phosphatases. Increase in farnesol should be associated with an increased intracellular FPP level. FPP is, in turn, converted from geranyl diphosphate (GPP), whose precursors are IPP and DMAPP. IPP and DMPP are end products of MEP pathway that exists in
E. coli. Conversion to FPP from IPP or DMPP requires ispA (or m-ispA). Following this context, we speculate that E. coli could produce farnesol better than the counterpart control cells under the up-regulated cellular conditions of an increased intracellular MEP pathway enzymes by metabolic engineering in combination with the special enzyme that converts IPP or DMAPP into FPP.
Gas Chromatography/Mass(GC/MS)

Efficient export of geraniol from E. coli to the media
We introduce an activator gene of AcrAB-TolC efflux pump (marA) to release the geraniol from the cells and increase the content in the media that shows increase these flavors in the "Flavolator". In our study, we confirmed that overexpressing of marA gives host E. coli high resistance against geraniol and reduce intracellular geraniol concentration.
 [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1653020 BBa_K1653020]
[http://parts.igem.org/wiki/index.php?title=Part:BBa_K1653020 BBa_K1653020]
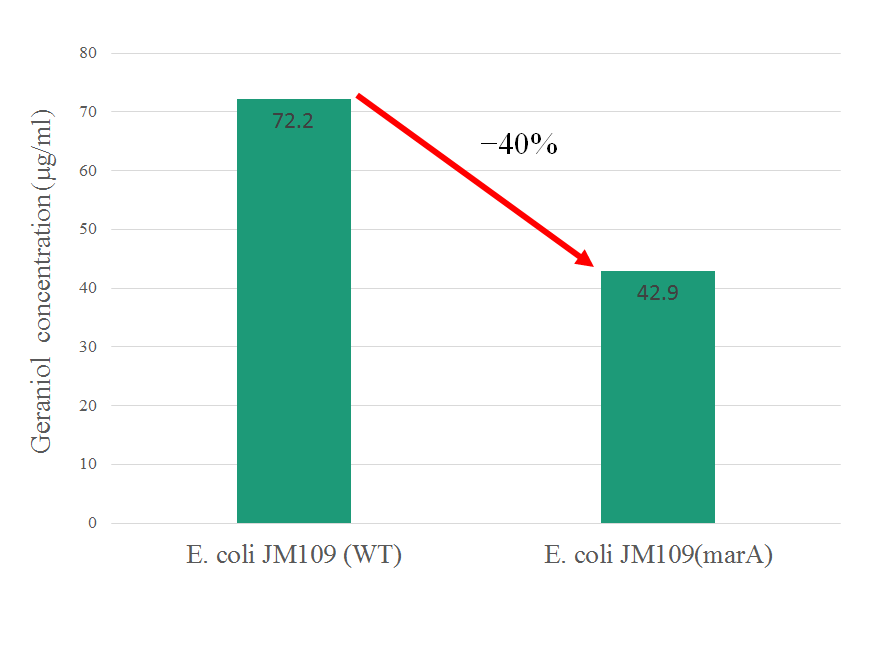
In this figure, intracellular content of geraniol was less in the strain E. coli JM109 (marA) than the strain E. coli JM109 (WT). The concentrations of intracellular geraniol from E. coli JM109 (marA) was 42.9 μg/ml, which was 40% lower than that from of E. coli JM109 (WT), 72.2 μg/ml. This figure is suggesting that internalized geraniol could be more efficiently exported through AcrAB-TolC efflux pump following the presumed activation of this gene by introducing the activator marA gene.
Enhancement of geraniol resistance
 [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1653020 BBa_K1653020]
[http://parts.igem.org/wiki/index.php?title=Part:BBa_K1653020 BBa_K1653020]
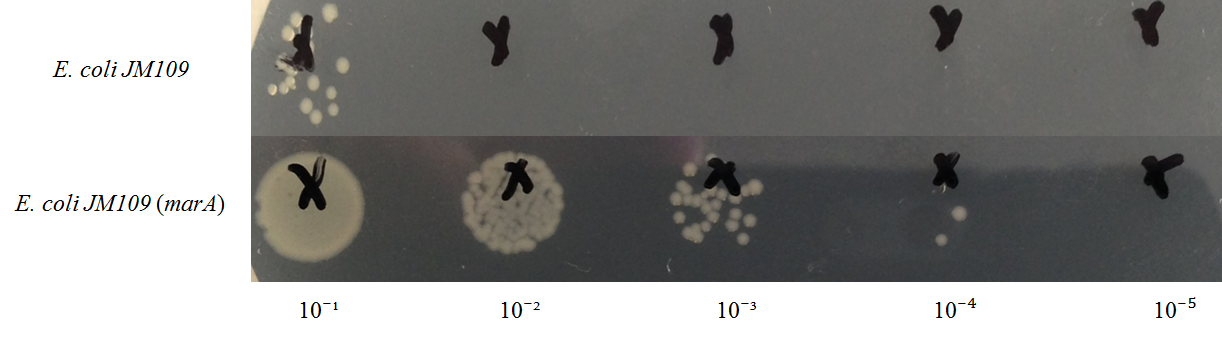
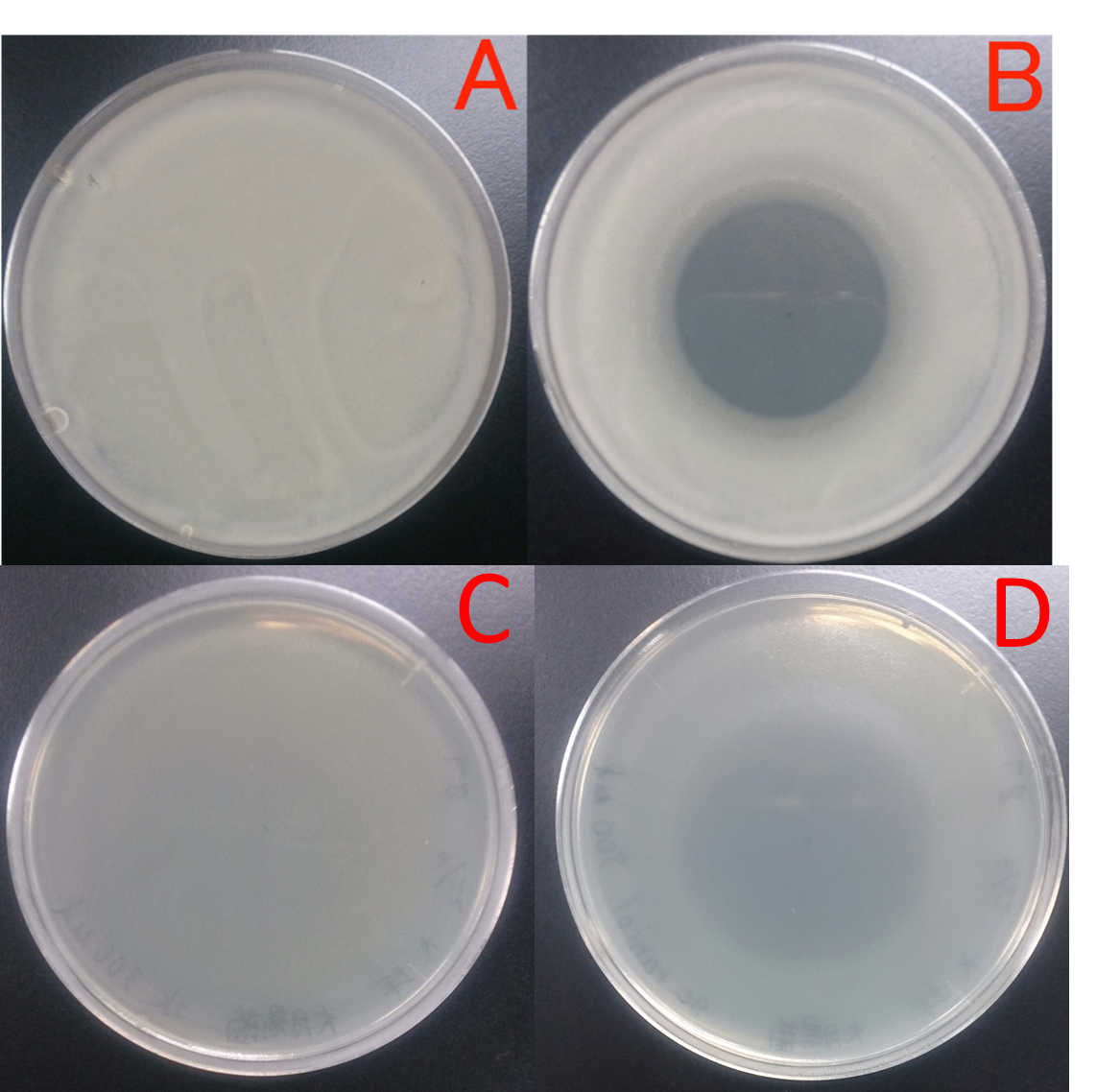
E. coli JM109 and E. coli JM109 (marA) were spotted on LBGMg agar plates in serial ten-fold dilutions (10⁻¹~10⁻⁵), overlaid with 1.0 % (v/v) geraniol hexane solution (geraniol solution), and incubated at 30°C for 24 h. This figure shows that E. coli JM109 (marA) cells that overexpress the marA product is more survived on 1.0 % geraniol solution overlay plates than the counterpart control E. coli JM109 wild type cells.
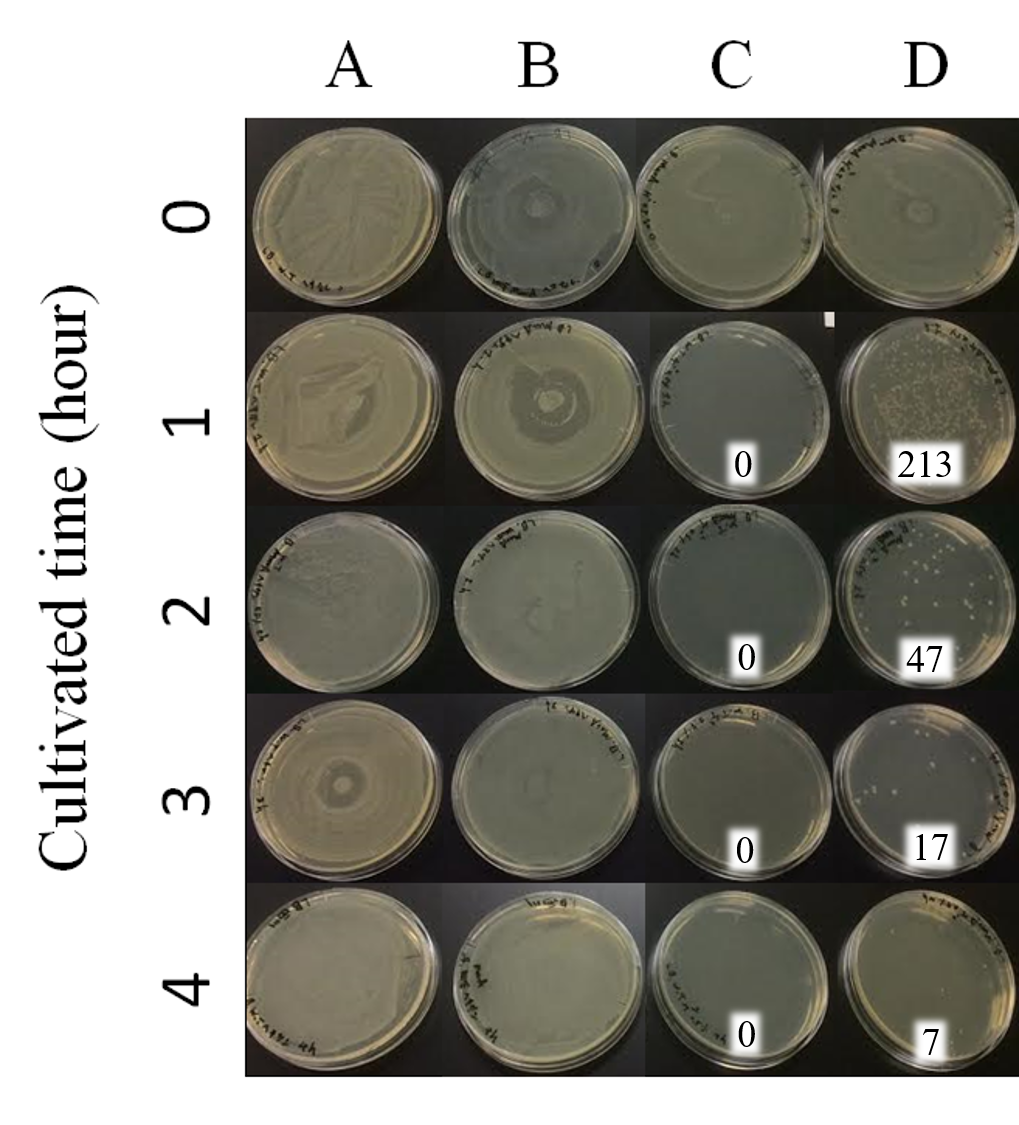
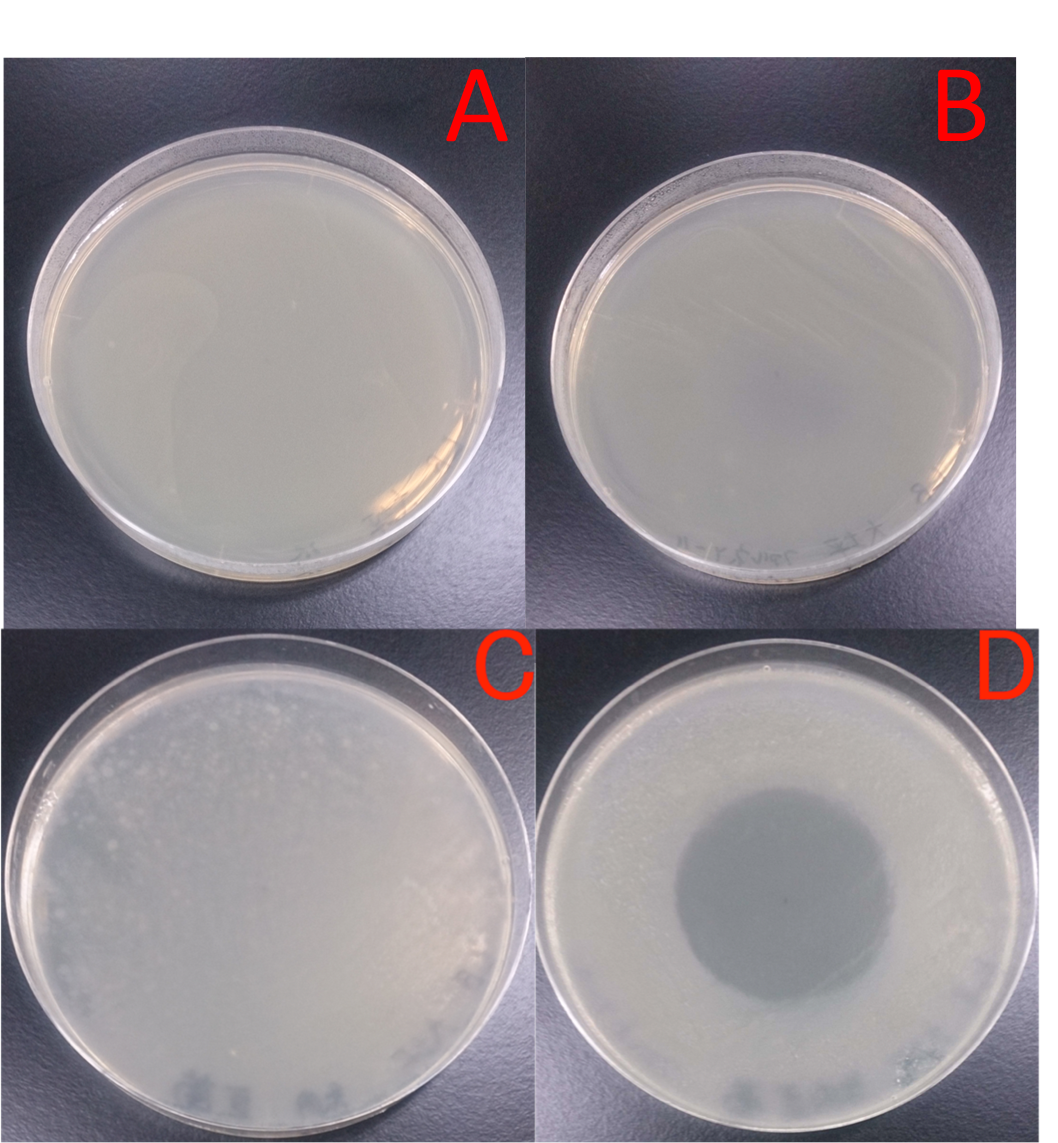
Time interval for treatment was set every 1 hour from 1 hour to 4 hours. A: E. coli JM109 (WT) + hexane; B: E. coli JM109 (marA) + hexane; C: E. coli JM109 (WT) + 0.5 % geraniol solution; D: E. coli JM109 (marA) + 0.5 % geraniol solution. As shown in Figs. 14 A and B, treatment with hexane of E. coli JM109 (WT) and of E. coli JM109 (marA) showed similar colony numbers during these treatment intervals to those of time zero. This result suggests that hexane at this concentration and duration of time for 4hours did not affect both cell growth. In contrast, treatment with geraniol of E. coli JM109 (WT) and of E. coli JM109 (marA) showed toxicities to both strains (Figs. 14 B, C and D). If we watch the colony numbers carefully, E. coli JM109 (marA) had more than E. coli JM109 (WT) during these treatment intervals ((Figs. 14 C and D). These results demonstrate that toxicity of the geraniol was less to the strain E. coli JM109 (marA) than the strain E. coli JM109 (WT).