Difference between revisions of "Team:EPF Lausanne/Project/Description"
| Line 180: | Line 180: | ||
<h2>The logic gate</h2> | <h2>The logic gate</h2> | ||
<div class="row"> | <div class="row"> | ||
| − | <div class="col-md- | + | <div class="col-md-2"> |
| − | <a href><img src="https://static.igem.org/mediawiki/2015/3/36/EPF_Lausanne_Project_Description_NAND_Truth_Table.jpg" style="width: | + | <a href><img src="https://static.igem.org/mediawiki/2015/3/36/EPF_Lausanne_Project_Description_NAND_Truth_Table.jpg" style="width:90%"></a> |
</div> | </div> | ||
| − | <div class="col-md- | + | <div class="col-md-10"> |
<p>In digital circuits, transistors are assembled to form <b>logic gates</b> [6], which can then be linked to form complex circuits. Based on this, we decided to assemble our bio-transisors to form a <b>(bio)logic gate</b>. Below is our design for the <b>NAND gate</b> [7].</p> | <p>In digital circuits, transistors are assembled to form <b>logic gates</b> [6], which can then be linked to form complex circuits. Based on this, we decided to assemble our bio-transisors to form a <b>(bio)logic gate</b>. Below is our design for the <b>NAND gate</b> [7].</p> | ||
</div> | </div> | ||
Revision as of 10:02, 18 September 2015
Bio LOGIC in 12 questions
What does Bio LOGIC stand for?
It stands for "Bio Logic Orthogonal gRNA-Implemented Circuit”. In a few words, we are working on implementing digital-like circuits in cells using dCas9.
Don’t biological circuits already exist?
Yes. However, difficulties in the multiplication and chaining of logic elements has hindered the complexification of these circuits. To overcome these limitations, an ideal in vivo logic element should be modular, reusable and orthogonal - i.e avoiding cross-talk with its host organism and the other elements of the circuit.
So, what’s different about your system?
We can avoid some of these issues by making a completely synthetic biological circuit. This is what we are doing by using the newly discovered dCas9 as a synthetic transcription factor.
How can dCas9 be used as a transcription factor?
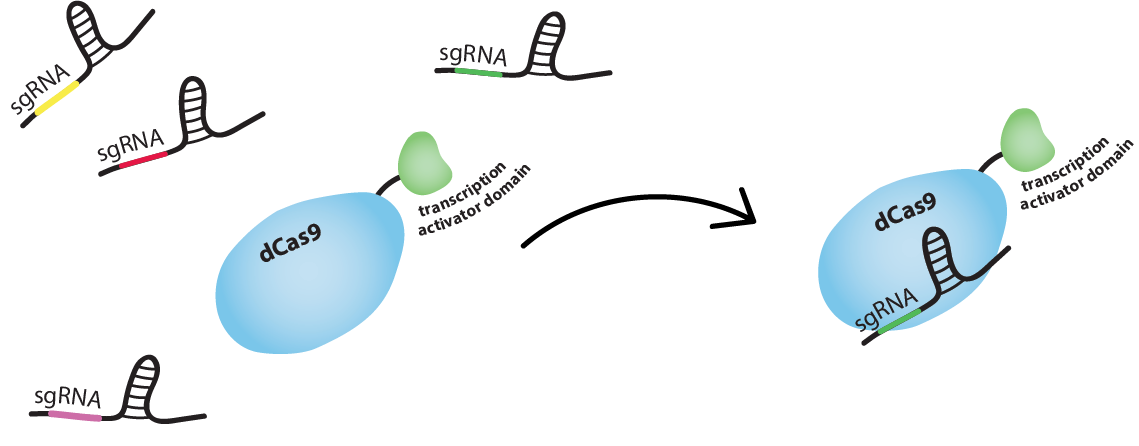
Well, you know CRISPR-Cas9, the RNA-guided DNA endonuclease, right? (Check out the Background tab for more information about CRISPR-Cas9.) We are using dCas9, the catalytically dead version of Cas9, which lacks the ability to cleave DNA. We will fuse dCas9 to a RNA Polymerase (RNAP) recruiting element. Depending on where it binds, this complex will either activate or inhibit transcription.
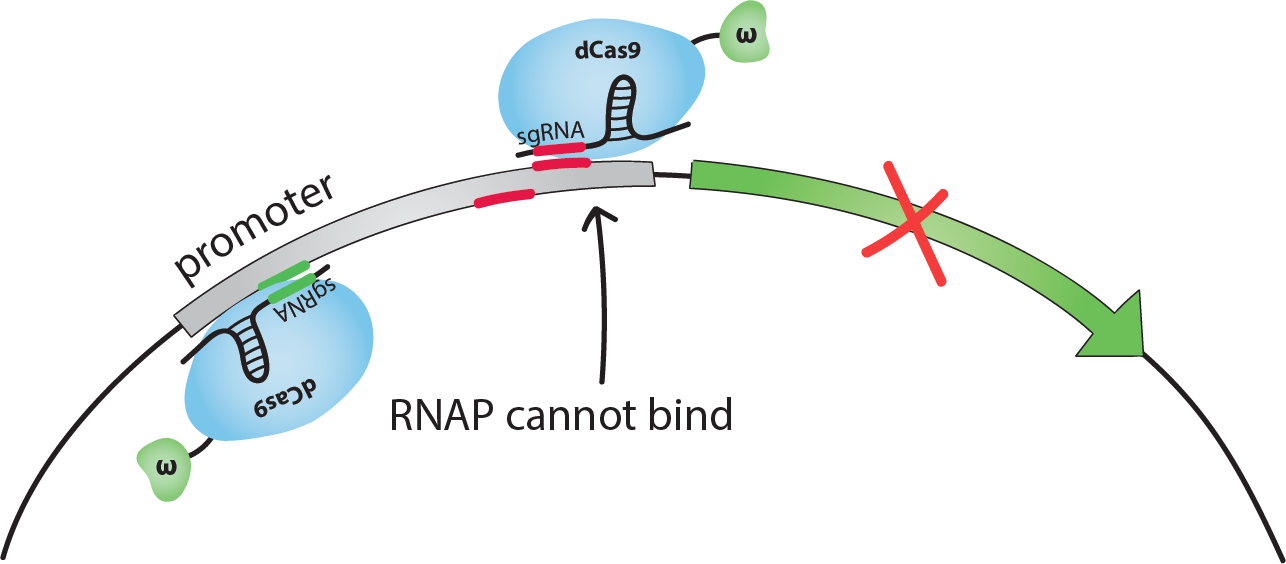
How does this activation/inhibition system work?
When dCas9 binds at an optimal distance upstream from the promoter, a RNAP recruiting elements with which it is fused will, in fact, recruit RNAP, thus activating the transcription of the gene that is controlled by this promoter. However, when dCas9 binds to the promoter, it will sterically hinder RNAP from binding at the transcription starting site, thus inhibiting the transcription of the gene.
How do you guide the dCas9 to activating/inhibiting region?
dCas9 works just like Cas9, meaning it is RNA-guided. Guide RNA (gRNA) and dCas9 can form a complex. This complex will bind tightly to a DNA sequence which is complementary to the gRNA. So we can guide dCas9 to activating or inhibiting regions of a promoter by producing gRNAs complementary to these sequences.
Can a gRNA-dCas9 complex activate one region and inhibit another in the same cell?
Yes, a gRNA-dCas9 complex will bind to any sequence that is complementary to the gRNA. So, if the activating region and the inhibiting region of different promoters have the same sequence, two identical gRNA-dCas9 complexes can bind both at the same time. This is also why we have to be careful not to target regions that are present in the genome of the host organism, to not interfere with cell’s standard function.
What happens if the activating region and the inhibiting region of the same promoter are bound by dCas9 at the same time?
That is a very good question! It was shown that inhibition is dominant in yeast (S. cerevisiae) [1]. This means that, if both activating and inhibiting regions are bound, the transcription of the gene will be inhibited. One goal of our project is to find out if this is also the case in bacteria (E. coli). We will also test our system in yeast.
How are you going to use this to make biological circuits?
Because of time constraints, we won’t be able to make a real biologic circuit. We aim to make and characterize a biological equivalent to the simplest element in a digital circuit, a transistor. Transistors function like controllable switches for electric current. Our bio-transistors work like switches for the transcription of a gene and can be assembled to form biological circuits.
What does your bio-transistor look like?
The bio-transistor is a synthetic promoter with a gene output, but regulated in a novel way. We insert this synthetic sequence in a cell, along with a gene producing dCas9 fused to the RNAP recruiting element and the sequence that produces an gRNA complementary to either the activating or the inhibiting region of the promoter. dCas9 and the gRNA are produced, they form a complex which binds to the activating or inhibiting region of the promoter, thus “turning the gene on or off”. Much like electronic PNP transisors, we may use steric inhibition to force the transistor into the "off" state even when it should have been activated.
How do you make biological circuits from bio-transistors?
Well, digital circuits are made out of logic gates, elements that perform basic logic functions (AND, OR, NOT, NOR, NAND, XOR, etc.), and logic gates are made out of transistors. Our idea is to assemble our bio-transistors into logic gates. By linking the output of one logic gate to the input of another one, we can make biological circuits that function in the same way as a digital circuit.
Sounds cool! But what can this be used for?
A single transistor is not very useful. However, by assembling a certain number of bio-transistors, we could make complex biological circuits that would have different outputs depending on many inputs. For example, we could make complex biosensors by building a circuit that is activated by the presence of a specific combination of molecules, or that has a different response for different combinations of molecules. This is only one example among the many applications of biological circuits.
Find out more about how we implemented our system in E. coli and in S. cerevisiae or about what’s already been done with biological circuits and the publications that inspired us, or check out our results.
References
[1] Farzadfard, F., Perli, S. D., Lu, T. K. (2013). Tunable and Multifunctional Eukaryotic Transcription Factors Based on CRISPR/Cas. ACS Synth. Biol., 2 (10), pp 604–613.
Design in E. Coli
The synthetic transcription factor: dCas9-ω
We will use the DNA-binding activity of the catalytically 'dead' version of Cas9, dCas9, to regulate genes.
For activation to be possible, dCas9 needs to be fused to a RNA polymerase (RNAP) recruiting element. We fused the ω (omega) subunit of RNAP to dCas9 [1]. The ω subunit works as a RNAP recruiting element in E. coli when working in a strain in which RNAP lacks the ω subunit. We used JEN202, "an E. coli MG1655 mutant in which rpoZ, encoding for the ω subunit of RNAP, was replaced by a spectinomycin resistance gene" [1], for all fluorescence measurements.
We used single guide RNAs (sgRNA) to guide dCas9-ω to targetted regions. An sgRNA "comprises a complementary domain that binds to the DNA followed by a “handle” that is bound by dCas9" [3]: in our case dCas9-ω. dCas9-ω and an sgRNA form a complex which will tightly bind a DNA sequence complementary to the 'complementary domain' of the sgRNA.
Find out how we regulate genes with dCas9-ω below!
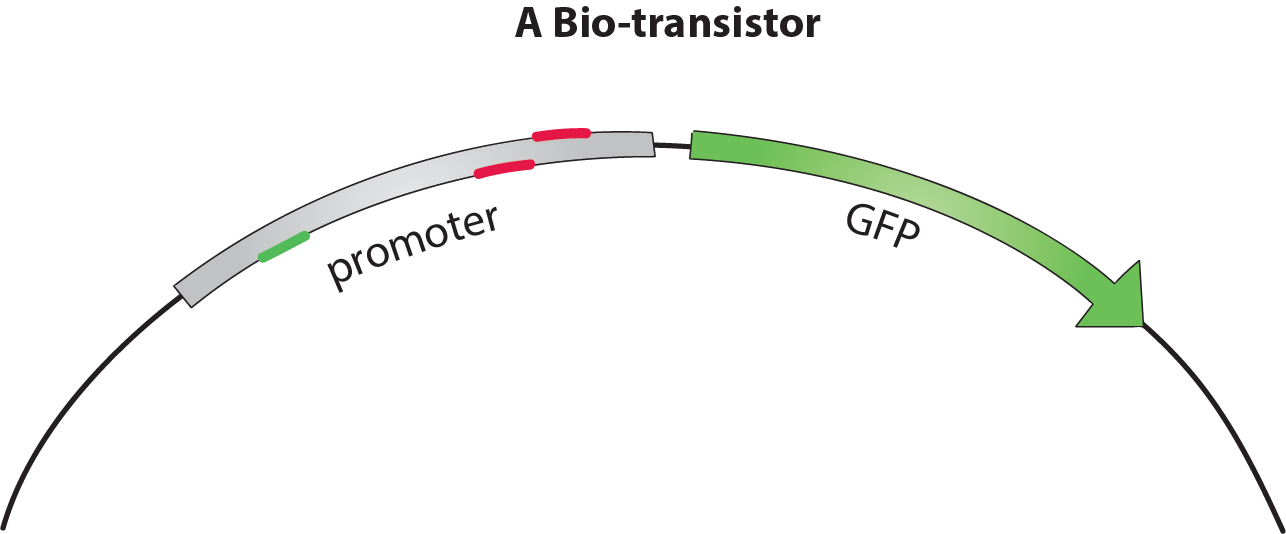
The bio-transistor
The bio-transistor for E. coli consists of a gene controlled by a synthetic promoter.
We synthesized promoters based on the constituve promoter J23117 [1]. This promoter consists of BBa_J233117, preceded by a protospacer-adjacent motif (PAM) rich upstream regulating sequence (URS) [4].
To show that the bio-transistor works with different promoter sequences, we tested our system for the promoters J23117 and J23117_alt. The sequence for J23117_alt was randomely generated, except for the -35 and -10 regions that were conserved from J23117. Some of our team members wrote a program in C++ and then in Python that generated this type of random sequence while conserving part of an original sequence.To observe the activity of a single bio-transistor, we used a gene encoding for green fluorescent protein (GFP) as a reporter gene.
If dCas9-ω guided by an sgRNA binds on the promoter close to the transcription start site, the binding of RNAP to DNA is sterically inhibited, and transcription is repressed. We chose to use sgRNAs complementary to sequences 14 or 18 bp upstream from the transcription start site (TSS). We will call these the inhibiting sites -14 and -18.If dCas9-ω guided by an sgRNA binds at an optimal distance upstream from the promoter, the ω subunit recruits RNAP and transcription is activated. We chose to use sgRNAs complementary to the sequence 71 bp upstream from the TSS. [1]Note that the activating sgRNA and the -18 inhibiting sgRNA are complementary to the bottom strand of the promoter, whereas the -14 inhibiting sgRNA is complementary to the top strand.When generating sequences for the sgRNAs and the binding sites, it is important that they are not present in the host organism's genome to avoid regulating genes other than the targetted one.
If both one inhibiting and the activating sgRNA for the same promoter are present in the same cell, we foresaw 2 possible situations. We simulated both with our model: either dCas9-ω can bind both the activating and the inhibiting sites, in this case the model predicts that inhibition will tend to be stronger, or dCas9-ω cannot bind both sites due to sterical hindrance in which case the overal effect should tend to be activating.For more information on these situations, check out our Modeling page, or see how this turned out experimentally in our Results!
We constructed plasmid pdCas9-ω, encoding for dCas9-ω controlled by a Tetracylcine-inducible promoter, from pdCas9-bacteria [2] and pWJ66 [1]. We synthesized 'sgRNA expressing cassettes' (IDT) controlled by the constitutive promoter pBad and inserted these into pdCas9-ω. We inserted either 1 or 2 sgRNAs (combinations of 1 activating and 2 inhibiting, affecting the same promoter).We used pWJ89 [1], GFP controlled by the J23117 promoter, [1] as the first 'reporter-transistor'. We constructed pWJ89_alt, GFP controlled by the J23117_alt promoter, from pWJ89 and J23117_alt (synthesized by IDT), and used it as our second 'reporter-transistor'.Find out more about the construction of these plasmids in our Lab Notebook!
We transformed JEN202 cells with one of the 'reporter-transistors' and pdCas9-ω with sgRNAs complementary to the activating and/or inhibiting regions of the promoter of the transistor, and measured the fluorescence of these cells with a plate reader or by flow cytometry. After trying several concentrations of Anhydrotetracycline (ATc), we decided to do all further experiments with 1 ng/mL ATc.Take a look at our results here!
Inducible bio-transistors
In a biological circuit, the production of the sgRNAs would most likely be induced, instead of constitutive. To test this with our bio-transistors, we changed the promoter of the 'sgRNA expressing cassette' to make it inducible. We did this by inserting a 'sgRNA expressing cassette', controlled by constitutive promoter pBad, in a plasmid containing AraC, placing the pBad promoter next to AraC. When placed next to one another, AraC is a repressor of pBad, and pBad can be induced by adding Arabinose to the medium [5]. Thus, the production of this sgRNAs becomes inducible with Arabinose.
We constructed three plasmids in this fashion with sgRNAs complementary to the J23117_alt promoter: one with the activating sgRNA controlled by pBad/AraC, one with one of the inhibiting sgRNAs controlled by pBAd/AraC, and one with the same inhibiting sgRNA controlled by pBad/Arac and the activating sgRNA controlled by pBad (constitutive).Find out more about the construction of these plasmids in our Lab Notebook!
We transformed JEN202 cells with the 'reporter-transistor' with J23117_alt promoter, pdCas9-ω and one of the pBad/AraC constructs, and (again) measured the fluorescence of these cells with a plate reader or by flow cytometry. We kept ATc levels at 1 ng/mL and tested 0 mM, 0.1 mM, 1 mM, 10 mM Arabinose.See how this worked out on our results page.
Linking bio-transisors
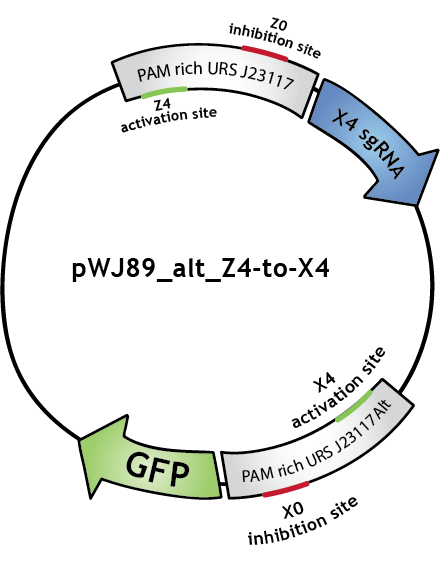
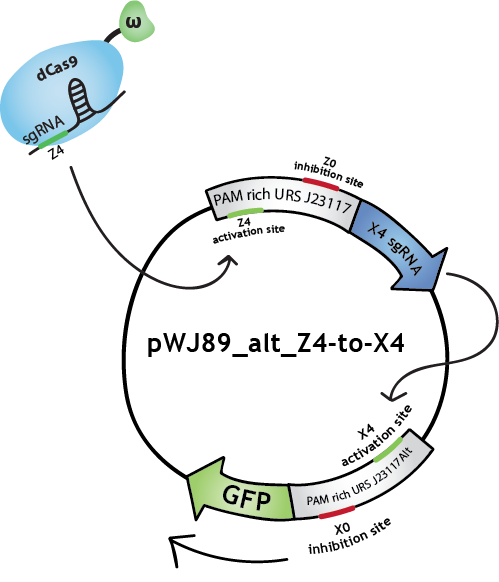
To make biological circuits with our bio-transistors, we will have to link several bio-transistors. To test whether the "signal" is strong enough for this to be possible with our system, we modified the transistor with promoter J23117 to express the sgRNA complementary to the activating site of J23117_alt, instead of GFP. In this way, when the first transistor (J23117) is activated by dCas9-ω bound to the corresponding sgRNA, another sgRNA will be expressed. This sgRNA, in complex with dCas9-ω, will bind the activating site of the second transistor (J23117_alt) which will activate the transcription of GFP. In an ideal situation, we would like to see that fluorescence levels obtained like this are close to levels obtained when simply activating one transistor.
We constructed this in one plasmid that we called pWJ89_alt_Z4-to-X4 by inserting the J23117 promoter followed by an 'sgRNA expressing cassette' (synthesized by IDT) into pWJ89_alt, the plasmid that contains GFP controlled by J23117_alt.You can find out more about the construction of this plasmid in our Lab Notebook!
We transformed JEN202 cells with pWJ89_alt_Z4-to-X4 and pdCas9-ω with different 'sgRNA expressing cassettes', notably the one expressing the sgRNA that will activate J23117. We kept ATc levels at 1 ng/mL.See the results here.
Note that in the Lab Notebook and in the figures the sgRNAs (and the corresponding binding sites on the promoters) have specific names:sgRNA activating J23117 and J23117_alt respectively: Z4 and X4. sgRNA -14 inhibiting J23117 and J23117_alt respectivly: Z0 and X0. sgRNA -18 inhibiting J23117 and J23117_alt respectively: Z35 and X35
The logic gate
In digital circuits, transistors are assembled to form logic gates [6], which can then be linked to form complex circuits. Based on this, we decided to assemble our bio-transisors to form a (bio)logic gate. Below is our design for the NAND gate [7].
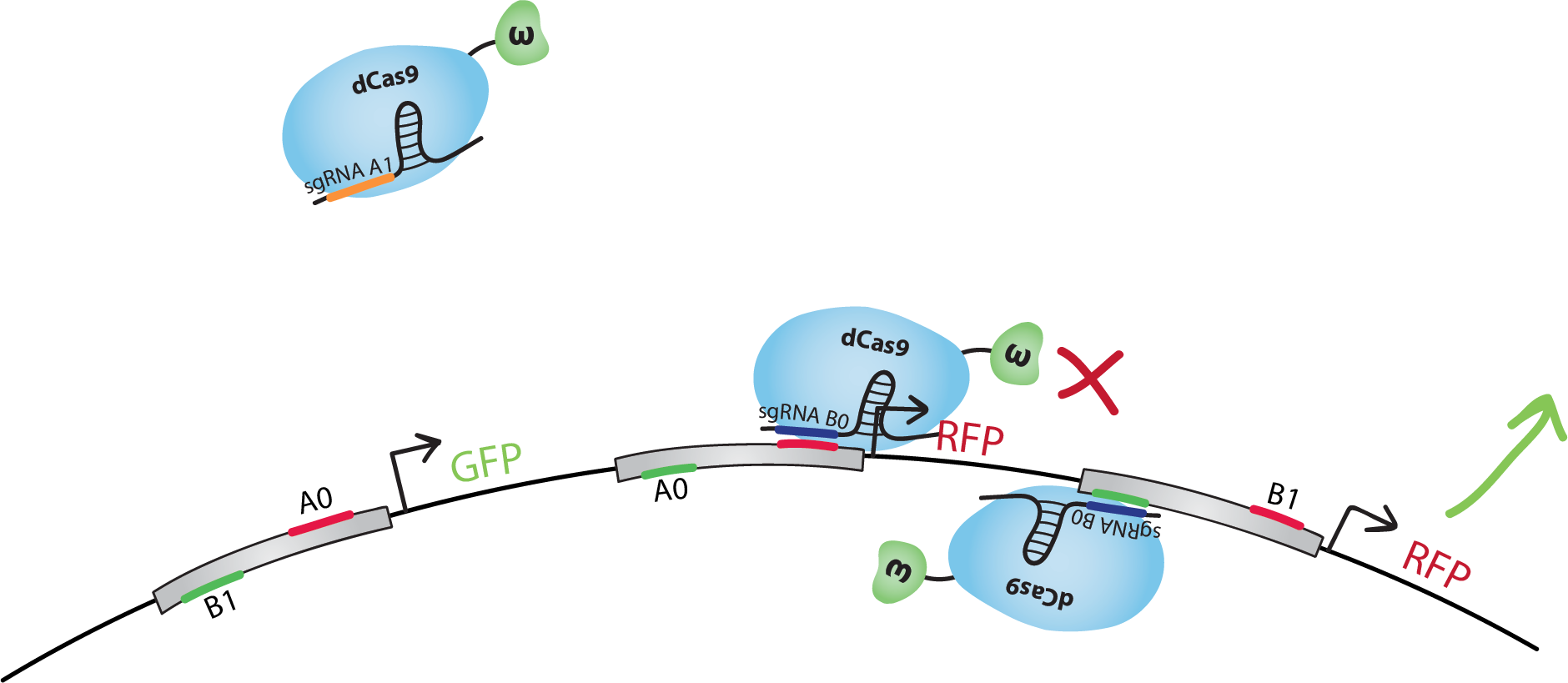
The design of our NAND gate contains 3 bio-transistors. The inputs, A and B, are in the form of sgRNAs called A0, A1, B0 and B1.Note that in our model the sequence A1 is not present in the transistors. dCas9-ω will bind to A1 but it will not find a binding site on the DNA.The output, C, is the transcription of GFP for C=0, and RFP for C=1.
We did the following design with the hypothesis that when activating and inhibiting sites of the same promoter are bound by dCas9-ω, the overall effect will be inhibition.Note that many other designs of this gate are possible, and that with similar systems we could also reproduce other gates, such as NOT, AND, OR, NOR, XOR, etc.
Let's go through the truth table together. Each case is illustrated on the right-hand side of this page.Let's start with A=0 and B=0. dCas9-ω will bind the inhibitory site of the 1st transistor. Transcription of GFP, C=0, will be at level 'i' (inhibited). dCas9-ω will bind both the inhibiting and the activating site of the 2nd transistor. Transcription of RFP(1), C=1, will be at level 'a/i' (activated and inhibited at the same time), which we suppose is equivalent to level 'i'. dCas9-ω will also bind to the activating site of the 3rd transistor. Transcription of RFP(2), C=1, will be at level 'a' (activated). If we consider 'i' levels of transcription to be negligeable, we have a final result of an 'a' level of RFP transcription, ie. we have C=1 just like in the truth table.In the case where A=0 and B=1: GFP is 'a/i'~='i', RFP(1) is 'a', RFP(2) is 'i'. Overall, we have an 'a' level of RFP transcription, so we have C=1, the desired result.In the case of A=1 and B=0: GFP is 'b' (basal) which we suppose is similar to level 'i', RFP(1) is 'i', RFP(2) is 'a'. Overall, we still have an 'a' level of RFP transcription, so we obtain C=1 like in the truth table.For the final state, A=1 and B=1: GFP is 'a', RFP(1) is 'b'~='i', RFP(2) is 'i'. Thus, we obtain an 'a' level of GFP transcription and C=0, following the NAND truth table.
Below is the 'biologic' version of the NAND truth table with trancription levels, summarizing the paragraph above.
| Input 1: A | Input 2: B | Output: [GFP]=0 [RFP1, RFP2]=1 | Output: C |
|---|---|---|---|
| 0 | 0 | [i] [a/i, a]~=[i, a] | 1 |
| 0 | 1 | [a/i]~=[i] [a, i] | 1 |
| 1 | 0 | [b]~=[i] [i, a] | 1 |
| 1 | 1 | [a] [b, i]~=[i, i] | 0 |
Due to time constraints, we were not able construct this gate and test it in the wet lab. However, modeling allowed us to qualitatively assess the functionality of logic gates in silico. After tuning some constants in order to reproduce basic activation and inhibition of a single transistor, we were able to reproduce more complex experiments (Chaining two transistors, and simutaneous activation and inhibiton). Finally, we were able to study the response of full logic gate, particularly the NAND gate.
References
[1] Bikard, D., Jiang, W., Samai, P., Hochschild, A., Zhang, F., & Marraffini, L. A. (2013). Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic acids research, 41(15), 7429-7437.[2] Qi, L. S., Larson, M. H., Gilbert, L. A., Doudna, J. A., Weissman, J. S., Arkin, A. P., & Lim, W. A. (2013). Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell, 152(5), 1173-1183.[3] Alec AK Nielsen & Christopher A Voigt (2014). Multi-input CRISPR/Cas circuits that interface host regulatory network. Molecular systems biology, 10(11), 763.[4] Addgene about protospacer-adjacent motif (PAM)[5]BBa_I0500: inducible pBad/AraC promoter[6] Wikipedia article on Logic gates[7]Wikipedia article on the NAND gate
| Electronic transistor | Bio LOGIC in S. cerevisiae | |
|---|---|---|
| Connector | Electrical wires | dCas9-VP64 protein |
| Transmitted information | Electrical voltage either high or low | gRNAs either activating or repressing |
| Receptor of information | Entry of next transistor, the base | Promoter CYC |
Elements of the transistor
Connector : dCas9-VP64
The dCas9 protein fused to a RNA Polymerase recruiting element, here VP64, can be used to activate or repress gene expression [1]. This regulation depends on the region of the promoter that dCas9-VP64 binds to.
Transmitted information : gRNAs
The gRNAs are sequences composed of a 20-nucleotide Specificity Determinant Sequence (SDS) and a 76-nucleotide scaffold. The SDS is complementary to a specific region of the promoter. When the site targetted by dCas9-VP64 is located at the beginning or before the promoter, the gene expression is enhanced. When the site targetted is located on the promoter and in particular on the TATA box or the TSS region, the gene expression is repressed [1].
We use the region of strongest activation and the region of strongest inhibition. The region of strongest activation is c3. Binding of dCas9-VP64 to region c3 produces a threefold increase of fluorescence. For the strongest inhibition we use c6 and c7 simultaneously. The reason is that a stronger inhibition is observed when two gRNAs bind the promoter. Binding of dCas9-VP64 to c6 and c7 produces a sevenfold decrease of fluorescence [1].
Each gRNA «cassette» is designed as in fig... : 5' - DsRed2 – polyA - HH ribozyme – gRNA SDS – gRNA scaffold – HDV ribozyme - 3'.
DsRed2 is a fluorescent protein. It acts as a reporter gene for the production of gRNAs [1].
The polyA tail is a 50-nucleotide-long sequence. It aims to stabilize [?????] the transcripted RNA and contributes to efficient mRNA progression away from the gene [5].
The hammerhead (HH) ribozyme and the hepatitis delta virus (HDV) ribozyme both carry out self-cleavage after transcription, [2] and [3].
The gRNA SDS is a 20-nucleotide sequence that guides dCas9 to a specific region on the promoter. Since we focus on three regions of the promoter, we use these three gRNA SDS : c3, c6 and c7. Each gRNA has four different exemplaries: c3_0, c3_1, c3_2, c3_3 for gRNA c3. The gRNAs c6 and c7 have the same notation. Each of these sequences are randomly generated by two programs in order to respect two conditions. Firstly, the
Receptor : CYC promoter
A key challenge in engineering transcriptional networks is to design orthogonal transcription factors or promoters. This means gRNAs must not interfere with one another. It has been shown that a single base-pair mismatch between the gRNA SDS and the promoter region is sufficient to prevent the binding of dCas9-VP64 [1]. We ensured to avoid cross-talking issues by synthesizing gRNA SDS that differ by at least 10 nucleotides out of 20.
Transistor
Logic gate
References
[1] Farzadfard F, Perli SD, Lu TK. Tunable and Multifunctional Eukaryotic Transcription Factors Based on CRISPR/Cas. ACS Synth Biol. 2013 Sep 11. 10.1021/sb400081r PubMed 23977949
[2] Gao Y, Zhao Y. Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol. 2013 Dec 30. doi: 10.1111/jipb.12152. 10.1111/jipb.12152 PubMed 24373158
[3] Nissim L, Perli SD, Fridkin A, Perez-Pinera P, Lu TK. Multiplexed and Programmable Regulation of Gene Networks with an Integrated RNA and CRISPR/Cas Toolkit in Human Cells. Mol Cell. 2014 May 14. pii: S1097-2765(14)00355-4. doi: 10.1016/j.molcel.2014.04.022. 10.1016/j.molcel.2014.04.022 PubMed 24837679
[4] ...
[5] Dower K1, Kuperwasser N, Merrikh H, Rosbash M. A synthetic A tail rescues yeast nuclear accumulation of a ribozyme-terminated transcript. RNA. 2004 Dec;10(12):1888-99. PubMed 15547135.
Background
Programmable cells - Promises and Limitations
The ability to process information is fundamental to life. Cells use complex gene regulatory networks to effectively respond to the myriad of signals they receive from both the outside environment and their internal metabolism. This information processing capability enables them to move around, communicate, reproduce - in one word, survive.
Since the early 2000s, researchers have sought to harness this capability by re-engineering cellular signal processing pathways for various biotechnology applications. By implementing rational, controllable logic elements in cells, researchers aim to transform living systems into engineered "machines" that may perform functions ranging from industrial production to biomedical therapies, bioremediation and energy production [1].
Up to date, various strategies and genetic parts have been used to implement information processing systems in cells. Many of these designs use the regulation of DNA expression as the adjustable signal in the circuit. A good example is the “repressilator” built by Elowitz et al. in 2000 that uses three orthogonal repressor-promoter pairs : LacI, tetR and λ cI [2]. Since then, multiple other repressors and activators (zinc-fingers, TALEs) have been used to target specific DNA sequences. Additional recent strategies to engineer synthetic biological circuits include the control of RNA stability, RNA translation or protein-protein interactions as the basis for signal transmission.
The challenge today is to transition from the creation of small genetic “devices” to cells that efficiently perform logical functions that effectively mimic those present in electronic circuits. This challenge calls for innovative strategies and rational design [3]. The engineering-driven approach of synthetic biology, including parts standardization, modular components, modeling and systematic strategies to create biological with reliable and predictable behaviors - holds part of the solution. The other key to the problem is to increase the number of usable orthogonal genetic components for signal processing [4]. The characterization of parts that do not cross-talk with the cellular machinery nor other parts of the genetic circuit promises to improve circuit robustness and efficiency.
Indeed, without orthogonal parts, retroactivity may limit circuit efficiency. Retroactivity involves the risk of downstream genetic elements interfering with upstream ones. Such interference is especially problematic when one tries to connect multiple elements in complex circuits. Retroactivity is not the only limitation of current cell signal processing circuits. To begin with, such circuits are slow : their response time can be measured in hours or even days. Furthermore, such circuits are often unreliable and offer low signal-to-noise ratios. Low output signal may make the connecting of multiple circuits impossible. Another limiting factor is the fact that the transcription factors used as fundamental parts for the circuit may be toxic for the cell and that the circuit itself may monopolize the cell’s resourcer and alter its behaviour. Finally, circuit parts may behave unexpectedly in new genetic contexts [5]. These issues underline the huge task at hand when one dreams of transferring computing from a world of silicon into the realm of biology.
dCas9 to the rescue!
CRISPR stands for Clustered, regularly interspaced, short palindromic repeat arrays. It plays the role of an 'immune system' for bacteria by targeting and degrading foreign DNA. The CRISPR systems uses a Cas9 (CRISPR-associated) nuclease to introduce double-strand breaks to the DNA sequences that are complementary to “guide” RNA (gRNA) [6]. Catalytically “dead” Cas9 (dCas9) lacks the ability to cleave DNA and may act as programmable transcription regulator by either preventing the binding of the RNA polymerase (RNAP) to the targeted DNA - or as an activator when fused to a RNAP recruiting element (the omega (ω) subunit of RNAP in E. Coli and VP64 in Yeast) [7].
The main advantage of this system is that many different orthogonal pairs of synthetic promoters and targeting gRNAs can be designed. In addition, synthetic promoters may be designed in order to have multiple regulating binding sites and thus be able to have multiple inputs, both activating and inhibiting. Since the use of the CRISPR technology to regulate gene expression is relatively new, few logic circuits have been tested with this technique (NOT gates are the most complex circuits reported in the literature) [8]. The challenges with this system are dCas9 toxicity, non-specific targeting of dCas9 and the use of Cas9 as a shared resource for the entire circuit.
However, given the numerous advantages of this system, we decided to attempt to represent binary signals (via transistors) and build logic gates with these two ”elementary” CRISPR/Cas9- dependent operations. We hope hope to demonstrate its viability as a transformative tool to rewire genetic networks.
References
[1] Brophy, J. A., & Voigt, C. A. (2014). Principles of genetic circuit design. Nature methods, 11(5), 508-520.[2] A Synthetic Oscillatory Network of Transcriptional Regulators; Michael Elowitz and Stanislas Leibler; Nature. 2000 Jan 20;403(6767):335-8.[3] Purnick, P. E., & Weiss, R. (2009). The second wave of synthetic biology: from modules to systems. Nature reviews Molecular cell biology, 10(6), 410-422.[4] Bradley, R. W., & Wang, B. (2015). Designer cell signal processing circuits for biotechnology. New biotechnology.[5] Brophy, J. A., & Voigt, C. A. (2014). Principles of genetic circuit design. Nature methods, 11(5), 508-520.[6] Sashital, D.G., Wiedenheft, B. & Doudna, J.A. Mechanism of foreign DNA selection in a bacterial adaptive immune system. Mol. Cell 46, 606–615 (2012)[7] Bikard, D., Jiang, W., Samai, P., Hochschild, A., Zhang, F., & Marraffini, L. A. (2013). Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic acids research, 41(15), 7429-7437.[8] Qi, L.S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).