Difference between revisions of "Team:Bielefeld-CeBiTec/Results/HeavyMetals"
| Line 24: | Line 24: | ||
</br> | </br> | ||
| − | The different sensors we worked with were characterized <i> in vivo </i>as well as <i>in vitro</i>.</br> </br> | + | <p>The different sensors we worked with were characterized <i> in vivo </i>as well as <i>in vitro</i>.</p></br> </br> |
| − | We tested the influence of each heavy metal on our sensors <i> in vivo </i> Therefore we used heavy metal concentrations based on heavy metal occurrences measured all over the world.</br> | + | <p>We tested the influence of each heavy metal on our sensors <i> in vivo </i> Therefore we used heavy metal concentrations based on heavy metal occurrences measured all over the world.</P></br> |
| Line 37: | Line 37: | ||
</figure> | </figure> | ||
</br> | </br> | ||
| − | </br>The tested heavy metal concentrations had no negative effect on <i>E. colis </i>growth. Moreover there is no significant difference between the curves with heavy metals and the controls. This first experiment showed us, in vivo characterization with these sensors under the tested heavy metal concentrations is possible. Most of our sensors were cultivated in the BioLector. Due to the accuracy of this device we could measure our sample in duplicates. </br></br> | + | </br> |
| + | <p<The tested heavy metal concentrations had no negative effect on <i>E. colis </i>growth. Moreover there is no significant difference between the curves with heavy metals and the controls. This first experiment showed us, in vivo characterization with these sensors under the tested heavy metal concentrations is possible. Most of our sensors were cultivated in the BioLector. Due to the accuracy of this device we could measure our sample in duplicates.</p> </br></br> | ||
| Line 58: | Line 59: | ||
</br></br> | </br></br> | ||
<h2><i>in vivo</i></h2></br></br> | <h2><i>in vivo</i></h2></br></br> | ||
| − | Our sensor for chromium detection consists of ChrB the repressor and the chromate specific promoter ChrP. The promoter is regulated by the ChrB, which binds Cr-ions. Behind the promoter is a sfGFP for detection of a fluorescence signal. | + | <p<Our sensor for chromium detection consists of ChrB the repressor and the chromate specific promoter ChrP. The promoter is regulated by the ChrB, which binds Cr-ions. Behind the promoter is a sfGFP for detection of a fluorescence signal. |
| − | <i>In vivo</i> we could show that the addition of different concentrations of chromium have different effects to transcription of sfGFP. </br></br> | + | <i>In vivo</i> we could show that the addition of different concentrations of chromium have different effects to transcription of sfGFP.</p> </br></br> |
<figure style="width: 600px"> | <figure style="width: 600px"> | ||
| Line 70: | Line 71: | ||
<figcaption>Fluorescence levels at three different stages of cultivation. Shown are levels after 60 minutes, 150 minutes and 650 minutes. Error bars represent the standard deviation of three biological replicates.</figcaption> | <figcaption>Fluorescence levels at three different stages of cultivation. Shown are levels after 60 minutes, 150 minutes and 650 minutes. Error bars represent the standard deviation of three biological replicates.</figcaption> | ||
</figure> | </figure> | ||
| − | </br>Our data lead to the conclusion that in a cell based system it is possible to detect chromium. | + | </br><p>Our data lead to the conclusion that in a cell based system it is possible to detect chromium. |
| − | In contrast to our expectations with higher chromium concentrations we got lower fluorescence levels. These observations needed further investigation. </br> | + | In contrast to our expectations with higher chromium concentrations we got lower fluorescence levels. These observations needed further investigation. </p></br> |
<h2><i>in vitro</i></h2> | <h2><i>in vitro</i></h2> | ||
| Line 106: | Line 107: | ||
<h2><i>in vivo</i></h2></br></br> | <h2><i>in vivo</i></h2></br></br> | ||
| − | Our sensor for copper detection consists of CueR a MerR like activator and the copper specific promoter CopAP. The promoter is regulated by CueR, which binds Cu2+-ions. We also used a sfGFP behind the promoter for detection trough a fluorescence signal. </br></br> | + | <p>Our sensor for copper detection consists of CueR a MerR like activator and the copper specific promoter CopAP. The promoter is regulated by CueR, which binds Cu2+-ions. We also used a sfGFP behind the promoter for detection trough a fluorescence signal.</p> </br></br> |
| Line 113: | Line 114: | ||
<figcaption>Time course of the induction of a copper biosensor with sfGFP for different copper concentrations in vivo. The data are measured with BioLector and normalized on OD600. Error bars represent the standard deviation of two biological replicates.</figcaption> | <figcaption>Time course of the induction of a copper biosensor with sfGFP for different copper concentrations in vivo. The data are measured with BioLector and normalized on OD600. Error bars represent the standard deviation of two biological replicates.</figcaption> | ||
</figure></br></br> | </figure></br></br> | ||
| − | <i>In vivo</i> we could show that the adding different concentrations of copper has effects on the transcription levels of sfGFP. </br></br> | + | <p><i>In vivo</i> we could show that the adding different concentrations of copper has effects on the transcription levels of sfGFP.</p> </br></br> |
<figure style="width: 600px"> | <figure style="width: 600px"> | ||
| Line 120: | Line 121: | ||
</figure></br> | </figure></br> | ||
| − | The shown data suggest that sensing copper with our device is possible even if the detectable concentrations are higher than the desireble sensitivity limits. Therfore we tested the copper sensor in our <i>in vitro</i> transcription translation approach. | + | <p>The shown data suggest that sensing copper with our device is possible even if the detectable concentrations are higher than the desireble sensitivity limits. Therfore we tested the copper sensor in our <i>in vitro</i> transcription translation approach.</p></br></br> |
| Line 131: | Line 132: | ||
<figcaption>Influence of different copper concentrations on our crude cell extract. Error bars represent the standard deviation of three biological replicates.</figcaption> | <figcaption>Influence of different copper concentrations on our crude cell extract. Error bars represent the standard deviation of three biological replicates.</figcaption> | ||
</figure></br> | </figure></br> | ||
| − | As shown above copper has no negatice influence on the functuality of our cell extact. Therefore a ralatively stable system for copper sensing is provided.</br></br></br> | + | <p>As shown above copper has no negatice influence on the functuality of our cell extact. Therefore a ralatively stable system for copper sensing is provided.</p></br></br></br> |
| + | |||
| + | |||
| + | <p>First Tests with specific cell extract and different copperconcentrations lead to further tests and normilisations</p></br> | ||
| − | |||
<!-- Induktion mit Kupfer im Kupfer spezifischen Extrakt --> | <!-- Induktion mit Kupfer im Kupfer spezifischen Extrakt --> | ||
<figure style="width: 600px"> | <figure style="width: 600px"> | ||
<a href="https://static.igem.org/mediawiki/2015/4/45/Bielefeld-CeBiTec_induction_copper_in_CueR_cell-extract.jpeg" data-lightbox="heavymetals" data-title="Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction of copper inducible promoter without T7 in front of the operator site with different copper concentrations. Error bars represent the standard deviation of three biological replicates. "><img src="https://static.igem.org/mediawiki/2015/4/45/Bielefeld-CeBiTec_induction_copper_in_CueR_cell-extract.jpeg" alt="Adjusting the detection limit"></a> | <a href="https://static.igem.org/mediawiki/2015/4/45/Bielefeld-CeBiTec_induction_copper_in_CueR_cell-extract.jpeg" data-lightbox="heavymetals" data-title="Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction of copper inducible promoter without T7 in front of the operator site with different copper concentrations. Error bars represent the standard deviation of three biological replicates. "><img src="https://static.igem.org/mediawiki/2015/4/45/Bielefeld-CeBiTec_induction_copper_in_CueR_cell-extract.jpeg" alt="Adjusting the detection limit"></a> | ||
<figcaption>Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction of copper inducible promoter without T7 in front of the operator site with different copper concentrations. Error bars represent the standard deviation of three biological replicates. | <figcaption>Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction of copper inducible promoter without T7 in front of the operator site with different copper concentrations. Error bars represent the standard deviation of three biological replicates. | ||
| − | </figure> | + | </figure> |
| + | |||
| + | <p>Fluorescences normalised on coppers influence to the cell extract are shown above.<p/> </br> | ||
<!--obrige Abbildung durch den errechneten Korrekturfaktor angepasst, da verschiedene Faktoren auf Zellextrakt wirken und so diesen beeinflussen.--> | <!--obrige Abbildung durch den errechneten Korrekturfaktor angepasst, da verschiedene Faktoren auf Zellextrakt wirken und so diesen beeinflussen.--> | ||
| Line 144: | Line 149: | ||
<a href="https://static.igem.org/mediawiki/2015/4/4c/Bielefeld-CeBiTec_correction_induction_copper_in_cueR_cell-extract.jpeg" data-lightbox="heavymetals" data-title="Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction of copper inducible promoter without T7 in front of the operator site with different copper concentrations. Error bars represent the standard deviation of three biological replicates. Data are normalized on coppers influence to the cell extract."><img src="https://static.igem.org/mediawiki/2015/4/4c/Bielefeld-CeBiTec_correction_induction_copper_in_cueR_cell-extract.jpeg" alt="Adjusting the detection limit"></a> | <a href="https://static.igem.org/mediawiki/2015/4/4c/Bielefeld-CeBiTec_correction_induction_copper_in_cueR_cell-extract.jpeg" data-lightbox="heavymetals" data-title="Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction of copper inducible promoter without T7 in front of the operator site with different copper concentrations. Error bars represent the standard deviation of three biological replicates. Data are normalized on coppers influence to the cell extract."><img src="https://static.igem.org/mediawiki/2015/4/4c/Bielefeld-CeBiTec_correction_induction_copper_in_cueR_cell-extract.jpeg" alt="Adjusting the detection limit"></a> | ||
<figcaption>Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction of copper inducible promoter without T7 in front of the operator site with different copper concentrations. Error bars represent the standard deviation of three biological replicates. Data are normalized on coppers influence to the cell extract.</figcaption> | <figcaption>Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction of copper inducible promoter without T7 in front of the operator site with different copper concentrations. Error bars represent the standard deviation of three biological replicates. Data are normalized on coppers influence to the cell extract.</figcaption> | ||
| − | </figure> | + | </figure></br> |
| + | <p>In addition to the native promoter operator device as measured above reporter constructs under the control of T7 promoter were tested.</p> </br> | ||
<!-- Es wurde auch das Konstrukt mit einen T7 davor eingesetzt, es zeichen sich unterschhiede inder Flurescens ausbeute, so mit ist für das CFPS system ein vorgeschalteter T7 sinnvoll zur besseren sensitivität des Systems. --> | <!-- Es wurde auch das Konstrukt mit einen T7 davor eingesetzt, es zeichen sich unterschhiede inder Flurescens ausbeute, so mit ist für das CFPS system ein vorgeschalteter T7 sinnvoll zur besseren sensitivität des Systems. --> | ||
| Line 150: | Line 156: | ||
<a href="https://static.igem.org/mediawiki/2015/c/ce/Bielefeld-CeBiTec_induction_T7-copAP_copper_in_cueR_cell-extract.jpeg" data-lightbox="heavymetals" data-title="Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction with different copper concentrations. Error bars represent the standard deviation of three biological replicates."><img src="https://static.igem.org/mediawiki/2015/c/ce/Bielefeld-CeBiTec_induction_T7-copAP_copper_in_cueR_cell-extract.jpeg" alt="Adjusting the detection limit"></a> | <a href="https://static.igem.org/mediawiki/2015/c/ce/Bielefeld-CeBiTec_induction_T7-copAP_copper_in_cueR_cell-extract.jpeg" data-lightbox="heavymetals" data-title="Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction with different copper concentrations. Error bars represent the standard deviation of three biological replicates."><img src="https://static.igem.org/mediawiki/2015/c/ce/Bielefeld-CeBiTec_induction_T7-copAP_copper_in_cueR_cell-extract.jpeg" alt="Adjusting the detection limit"></a> | ||
<figcaption>Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction with different copper concentrations. Error bars represent the standard deviation of three biological replicates.</figcaption> | <figcaption>Copper specific cell extract made from <i>E. coli</i> cells which have already expressed the activator before cell extract production. Induction with different copper concentrations. Error bars represent the standard deviation of three biological replicates.</figcaption> | ||
| − | </figure> | + | </figure> </br> |
| + | |||
| + | <p>Compared to the former fluorecence leves the T7 reporter device showed higer levels therefore a reporter device under the control of T7 promoter is more sutable for our CFPS.</p> </br> | ||
| + | |||
<!-- auch dieses Abbildung wurde mit dem Korrekturfaktor korrigiert--> | <!-- auch dieses Abbildung wurde mit dem Korrekturfaktor korrigiert--> | ||
| Line 156: | Line 165: | ||
<a href="https://static.igem.org/mediawiki/2015/0/01/Bielefeld-CeBiTec_correction_induction_T7-copAP_in_cueR_cell-extract.jpeg" data-lightbox="heavymetals" data-title="TEXT Error bars represent the standard deviation of three biological replicates."><img src="https://static.igem.org/mediawiki/2015/0/01/Bielefeld-CeBiTec_correction_induction_T7-copAP_in_cueR_cell-extract.jpeg" alt="Adjusting the detection limit"></a> | <a href="https://static.igem.org/mediawiki/2015/0/01/Bielefeld-CeBiTec_correction_induction_T7-copAP_in_cueR_cell-extract.jpeg" data-lightbox="heavymetals" data-title="TEXT Error bars represent the standard deviation of three biological replicates."><img src="https://static.igem.org/mediawiki/2015/0/01/Bielefeld-CeBiTec_correction_induction_T7-copAP_in_cueR_cell-extract.jpeg" alt="Adjusting the detection limit"></a> | ||
<figcaption>TEXT. Error bars represent the standard deviation of three biological replicates.</figcaption> | <figcaption>TEXT. Error bars represent the standard deviation of three biological replicates.</figcaption> | ||
| − | </figure> | + | </figure> </br> |
| + | <p> after normalising on coppers influcence to the cell extract these differecnces were even more pregnant</p></br> | ||
Revision as of 14:01, 14 September 2015
Heavy Metals
Zusammenfassung in ganz wenigen Worten.
The different sensors we worked with were characterized in vivo as well as in vitro.
We tested the influence of each heavy metal on our sensors in vivo Therefore we used heavy metal concentrations based on heavy metal occurrences measured all over the world.

Arsenic
in vivo
We tested an arsenic sensor with mRFP1 as reporter gene in vivo to confirm that the sensor is functional and test whether it is possible to detect the safety limit as defined by the WHO. We observed a reaction approximately five hours after addition of arsenic. The safety limit of 10 µg/L could clearly be distinguished from the negative control and the fluorescence signal increased up to a concentration of 500 µg/L. The signal in the presence of 1000 µg/L was slightly lower than in the presence of 500 µg/L.

in vitro
In order to test the arsenic sensor in our cell-free protein synthesis, we cloned a device that contains the arsenic operator between the T7 promoter and sfGFP with our optimized untranslated region (UTR). We tested this device in a cell extract that had been generated from cells expressing the arsenic repressor. We observed an induction when adding arsenic up to a concentration of 1.87 mg/L. As high arsenic concentrations inhibit the performance of the CFPS, we normalized the results for this effect. In the final application, this task is performed by our app.

Chromium
in vivo


Our data lead to the conclusion that in a cell based system it is possible to detect chromium. In contrast to our expectations with higher chromium concentrations we got lower fluorescence levels. These observations needed further investigation.
in vitro

As shown above copper has no negatice influence on the functuality of our cell extact. Therefore a ralatively stable system for copper sensing is provided.
First Tests with specific cell extract and different copperconcentrations lead to further tests and normilisations

Fluorescences normalised on coppers influence to the cell extract are shown above.

In addition to the native promoter operator device as measured above reporter constructs under the control of T7 promoter were tested.

Compared to the former fluorecence leves the T7 reporter device showed higer levels therefore a reporter device under the control of T7 promoter is more sutable for our CFPS.

after normalising on coppers influcence to the cell extract these differecnces were even more pregnant
Lead
in vivo


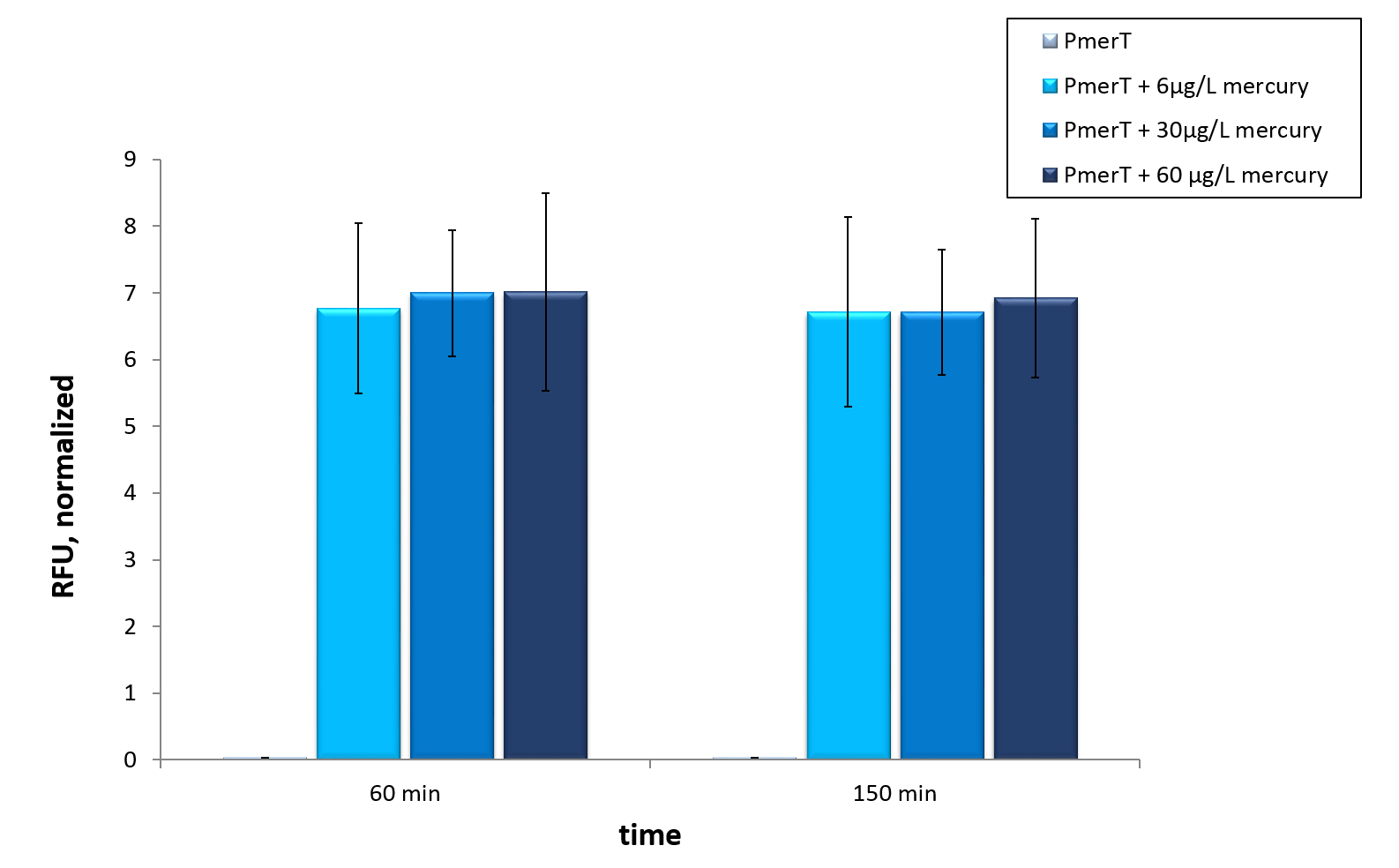
Mercury
in vivo


in vitro



Nickel
in vivo



