Difference between revisions of "Team:Bielefeld-CeBiTec/Project/PRIA"
m |
|||
| Line 26: | Line 26: | ||
<p>Our second approach to cell-free detection of heavy metals and date rape drugs is based on the interaction of an repressor that binds to a plasmid or another DNA fragment containing the operator sequence that is specifically recognized by the repressor protein. The repressor changes its conformation upon binding of the analyte and the bond to the DNA is disrupted. The disruption of this bond will be detected via a loss of a fluorescence signal caused by elution of labeled protein or DNA. We call this system <b>P</b>lasmid <b>R</b>epressor <b>I</b>nteraction <b>A</b>ssay (PRIA). </br> | <p>Our second approach to cell-free detection of heavy metals and date rape drugs is based on the interaction of an repressor that binds to a plasmid or another DNA fragment containing the operator sequence that is specifically recognized by the repressor protein. The repressor changes its conformation upon binding of the analyte and the bond to the DNA is disrupted. The disruption of this bond will be detected via a loss of a fluorescence signal caused by elution of labeled protein or DNA. We call this system <b>P</b>lasmid <b>R</b>epressor <b>I</b>nteraction <b>A</b>ssay (PRIA). </br> | ||
The advantage over the cell extract is that this method does neither require transcription nor translation of a reporter protein. Therefore, the signal can be measured much faster. For a similar system measurement of arsenic and cadmium contamination of water was possible within 15 to 30 minutes (<a href="https://2015.igem.org/Team:Bielefeld-CeBiTec/Project/PRIA#Siddiki2011">Siddiki <i>et al.</i> 2011</a>), thereby meeting one of the requirements often mentioned by potential users in our <a href="https://2015.igem.org/Team:Bielefeld-CeBiTec/StreetScience#speed" target="_blank"> survey</a> on synthetic biology and biosensors: speed. </br> | The advantage over the cell extract is that this method does neither require transcription nor translation of a reporter protein. Therefore, the signal can be measured much faster. For a similar system measurement of arsenic and cadmium contamination of water was possible within 15 to 30 minutes (<a href="https://2015.igem.org/Team:Bielefeld-CeBiTec/Project/PRIA#Siddiki2011">Siddiki <i>et al.</i> 2011</a>), thereby meeting one of the requirements often mentioned by potential users in our <a href="https://2015.igem.org/Team:Bielefeld-CeBiTec/StreetScience#speed" target="_blank"> survey</a> on synthetic biology and biosensors: speed. </br> | ||
| − | Furthermore, PRIA works with just two purified components, thereby minimizing the risk of releasing genetically modified organisms (GMOs) into the wild. Our system is not even subject to the regulations of the German "Gentechnikgesetz" (<a href="http://www.gesetze-im-internet.de/bundesrecht/gentg/gesamt.pdf" target="_blank">Genetic engineering Act</a>). This system should be robust against environmental factors, as long as they do not denaturize the protein and show increased stability and durability compared to conventional biosensors, since there are no metabolic pathways involved (Interview with | + | Furthermore, PRIA works with just two purified components, thereby minimizing the risk of releasing genetically modified organisms (GMOs) into the wild. Our system is not even subject to the regulations of the German "Gentechnikgesetz" (<a href="http://www.gesetze-im-internet.de/bundesrecht/gentg/gesamt.pdf" target="_blank">Genetic engineering Act</a>). This system should be robust against environmental factors, as long as they do not denaturize the protein and show increased stability and durability compared to conventional biosensors, since there are no metabolic pathways involved (Interview with Dr. Mathias Keller, district government of East Westphalia Lippe). It can be assumed that PRIA can still perform at high percentages of toxic substances, since neither translation nor transcription are required for the detection mechanism. |
</p> | </p> | ||
| Line 44: | Line 44: | ||
<h3>Immobilized Repressor</h3> | <h3>Immobilized Repressor</h3> | ||
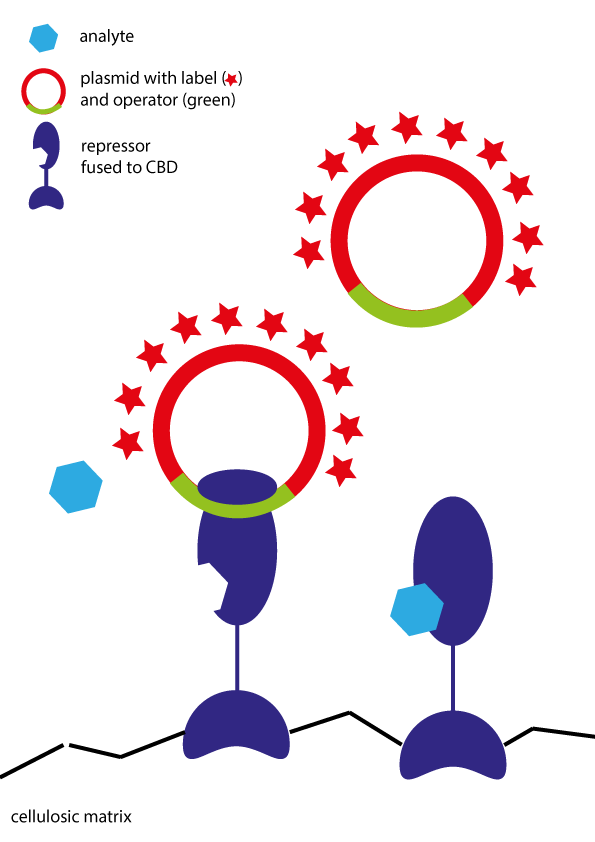
<p>Our approach was built on fusion proteins of the repressor proteins with cellulose binding domains (CBD). By adding these proteins to paper, we hoped to immobilize them in a functional form. Released labeled DNA was to be detected upon analyte addition.</p></br></br> | <p>Our approach was built on fusion proteins of the repressor proteins with cellulose binding domains (CBD). By adding these proteins to paper, we hoped to immobilize them in a functional form. Released labeled DNA was to be detected upon analyte addition.</p></br></br> | ||
| − | <figure> | + | <figure><a href="https://static.igem.org/mediawiki/2015/b/b9/PRIA_immobProtein_imagesonly.gif" data-lightbox="projectPRIA" data-title="Illustration of PRIA with repressor protein bound to a cellulose matrix. The signal measured is generated by the release of plasmid DNA." > |
| − | <img src="https://static.igem.org/mediawiki/2015/b/b9/PRIA_immobProtein_imagesonly.gif" alt="Sorry, cannot load this file at the moment" width="350 px"> | + | <img src="https://static.igem.org/mediawiki/2015/b/b9/PRIA_immobProtein_imagesonly.gif" alt="Sorry, cannot load this file at the moment" width="350 px"></a> |
<figcaption>Illustration of PRIA with repressor protein bound to a cellulose matrix. The signal measured is generated by the release of plasmid DNA.</figcaption> | <figcaption>Illustration of PRIA with repressor protein bound to a cellulose matrix. The signal measured is generated by the release of plasmid DNA.</figcaption> | ||
</figure> | </figure> | ||
| Line 52: | Line 52: | ||
<div class="Subtitle"> | <div class="Subtitle"> | ||
<h3>Immobilized DNA</h3> | <h3>Immobilized DNA</h3> | ||
| − | <p>The immobilization on ssDNA onto paper has been reported before (<a href="https://2015.igem.org/Team:Bielefeld-CeBiTec/Project/PRIA#Araújo2012">Araújo <i>et al.</i> 2012</a>). We adapted this method to enable the immobilization of dsDNA and combine it with an approach based on the measurement of the disruption of the binding of a GFP-tagged repressor protein to immobilized biotinylated DNA as reported before (Siddiki <i>et al.</i>). | + | <p>The immobilization on ssDNA onto paper has been reported before (<a href="https://2015.igem.org/Team:Bielefeld-CeBiTec/Project/PRIA#Araújo2012">Araújo <i>et al.</i> 2012</a>). We adapted this method to enable the immobilization of dsDNA and combine it with an approach based on the measurement of the disruption of the binding of a GFP-tagged repressor protein to immobilized biotinylated DNA as reported before (<a href="https://2015.igem.org/Team:Bielefeld-CeBiTec/Project/PRIA#Siddiki2011">Siddiki <i>et al.</i> 2011</a>). |
| − | <figure> | + | <figure><a href="https://static.igem.org/mediawiki/2015/d/d8/Bielefeld-CeBiTec_PT_PRIA_immobDNA.gif" data-lightbox="projectPRIA" data-title="Illustration of PRIA with immobilized DNA and a sfGFP-tagged repressor protein bound to it. The signal measured is generated by the release of the tagged protein."> |
| − | <img src="https://static.igem.org/mediawiki/2015/d/d8/Bielefeld-CeBiTec_PT_PRIA_immobDNA.gif" alt="Sorry, cannot load this file at the moment" width="350 px" align="middle"> | + | <img src="https://static.igem.org/mediawiki/2015/d/d8/Bielefeld-CeBiTec_PT_PRIA_immobDNA.gif" alt="Sorry, cannot load this file at the moment" width="350 px" align="middle"></a> |
<figcaption>Illustration of PRIA with immobilized DNA and a sfGFP-tagged repressor protein bound to it. The signal measured is generated by the release of the tagged protein.</figcaption> | <figcaption>Illustration of PRIA with immobilized DNA and a sfGFP-tagged repressor protein bound to it. The signal measured is generated by the release of the tagged protein.</figcaption> | ||
</figure></p> | </figure></p> | ||
Revision as of 09:05, 13 September 2015
Plasmid Repressor Interaction Assay
A cell-free detection system based on two purified components
Introduction
Our second approach to cell-free detection of heavy metals and date rape drugs is based on the interaction of an repressor that binds to a plasmid or another DNA fragment containing the operator sequence that is specifically recognized by the repressor protein. The repressor changes its conformation upon binding of the analyte and the bond to the DNA is disrupted. The disruption of this bond will be detected via a loss of a fluorescence signal caused by elution of labeled protein or DNA. We call this system Plasmid Repressor Interaction Assay (PRIA). The advantage over the cell extract is that this method does neither require transcription nor translation of a reporter protein. Therefore, the signal can be measured much faster. For a similar system measurement of arsenic and cadmium contamination of water was possible within 15 to 30 minutes (Siddiki et al. 2011), thereby meeting one of the requirements often mentioned by potential users in our survey on synthetic biology and biosensors: speed. Furthermore, PRIA works with just two purified components, thereby minimizing the risk of releasing genetically modified organisms (GMOs) into the wild. Our system is not even subject to the regulations of the German "Gentechnikgesetz" (Genetic engineering Act). This system should be robust against environmental factors, as long as they do not denaturize the protein and show increased stability and durability compared to conventional biosensors, since there are no metabolic pathways involved (Interview with Dr. Mathias Keller, district government of East Westphalia Lippe). It can be assumed that PRIA can still perform at high percentages of toxic substances, since neither translation nor transcription are required for the detection mechanism.
Aim
Our aim was to develop a sensor based on the disruption of the repressor-plasmid bond. Since paper-based systems are increasing in popularity because they are cheap and easily renewable, we wanted to establish a paper-based test strip, which enables swift detection of analytes by a loss of fluorescence signal.
Strategies
We started our experiments with a good characterized model system for repression and derepression: the lac operon and its repressor LacI. LacI immobilized on a Ni-NTA agarose column delivered the first proof of concept: Plasmid DNA containing the lac promoter was bound to LacI and could be detected in the elution fraction upon addition of Isopropyl-β-D-thiogalactopyranosid (IPTG) to the column. The protocol applied was similar to that of protein-protein interaction studies. There are two possible options for the transfer of this system from an impractical Ni-NTA agarose column to paper. The first one would be to immobilize the repressor protein on paper, the second is to immobilize the DNA with the operator on paper.
Immobilized Repressor
Our approach was built on fusion proteins of the repressor proteins with cellulose binding domains (CBD). By adding these proteins to paper, we hoped to immobilize them in a functional form. Released labeled DNA was to be detected upon analyte addition.
Immobilized DNA
The immobilization on ssDNA onto paper has been reported before (Araújo et al. 2012). We adapted this method to enable the immobilization of dsDNA and combine it with an approach based on the measurement of the disruption of the binding of a GFP-tagged repressor protein to immobilized biotinylated DNA as reported before (Siddiki et al. 2011).

Outview
We established the proof of concept for PRIA, and we showed that the immobilization of dsDNA on paper is feasible. We have sfGFP-tagged repressor proteins, so the next step will be to bring these aspects together and optimize a protocol that allows the establishment of the protein DNA complex on paper.
References
Araújo, Ana Caterina; Song, Yajing; Lundeberg, Joakim; Stahl, Patrik L.; Brumer, Harry (2012): Activated Paper Surfaces for the Rapid Hybridization of DNA through Capillary Transport. In Anal. Chem. 2012, 84, pp. 3311-3317. DOI: 10.1021/ac300025v
Lehtiö, Janne; Wernerús, Henrik; Samuelson, Patrik; Teeri, Tuula T.; Stahl, Stefan (2001): Directed immobillization of recombinant staphylococci on cotton fibers by functional display of a fungal cellulose-binding domain. In FEMS Microbiol Lett. 2001, 195(2), pp. 197-204. DOI: 10.1111/j.1574-6968.2001.tb10521.x 197-204
Siddiki, Mohammad Shohel Rana; Kawakami, Yasunari; Ueda, Shunsaku; Maeda, Isamu (2011): Solid Phase Biosensors for Arsenic or Cadmium Composed of A trans Factor and cis Element Complex.In Sensors 2011, 11, pp. 10063-10073. 10.3390/s111110063


