Difference between revisions of "Team:SJTU-BioX-Shanghai/Transport Module"
(→Western Blotting) |
|||
| Line 43: | Line 43: | ||
| figure = [[File:SJTUB dri4.jpg | 300px | frameless]] | | figure = [[File:SJTUB dri4.jpg | 300px | frameless]] | ||
| float = right | | float = right | ||
| − | |||
| id = 1.3.4 | | id = 1.3.4 | ||
| label = Western blotting result of the transformed strain PcpcG2-HR with wild type as the negative control. | | label = Western blotting result of the transformed strain PcpcG2-HR with wild type as the negative control. | ||
Revision as of 01:51, 19 September 2015
Contents
Basic design
Halorhodopsin (HR) proteins are light-driven inward-directed chloride pumps from halobacteria. They are membrane-integral proteins of the rhodopsin superfamily that form a covalent bond with the carotenoid-derived chromophore all-trans retinal. Absorption of a photon with a defined optimal wavelength induces trans-cis isomerization of retinal, which triggers a catalytic photocycle of conformational changes in the protein, resulting in the net import of one chloride per photon into the cytoplasm. (Amezaga, J. M. et al. 2014) We use it as our biodesalination driver which confers cyanobacteria the ability to absorb chloride to a significant degree.
As for sodium, cyanobacteria can actively export sodium ion to maintain low internal sodium concentration under saline environment. And this is mainly performed by Na+/H+ antiporter and P-type Na+ ATPase; the former one requires H+ gradient as the energy source and the latter one consumes ATP directly. Since the ATP synthase is driven by H+ gradient, we can simply regard that ATP is required for the active Na+ export. To achieve efficient biodesalination, we have to inhibit this mechanism. In our design of transport module, we create starvation condition to suppress active sodium export. Moreover, the negative membrane potential generated by Cl- that is absorbed by halorhodopsin would drive the influx of cation through sodium ion channels. (Amezaga, J. M. et al. 2014)
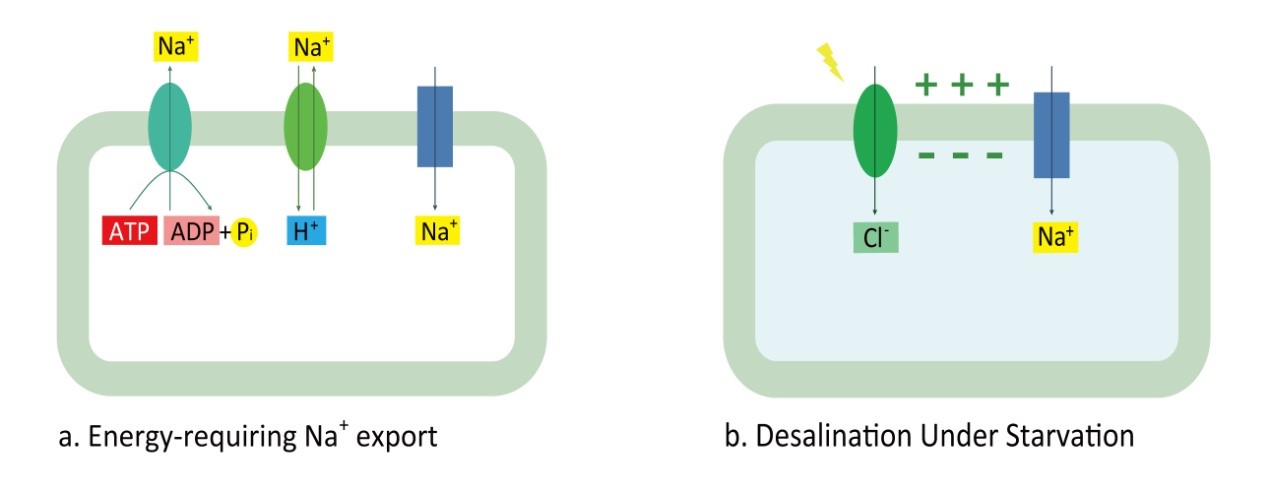
Figure 1.3.1 Design of transport module.
(a) Cyanobacteria actively export Na+ by Na+/H+ antiporter (green oval) and P-type Na+ ATPase (blue-green oval) to resist Na+ flowing inside through Na+ channels(Blue box) under saline environment. (b) Under starvation, ATP reserve has been exhausted and Na+ flow through Na+ channels, which is driven by the negative membrane potential generated by Cl- that is absorbed by halorhodopsin (green oval).
In summary, the halorhodopsin drives Cl- inside, then generates negative membrane potential which will drive the import of Na+ given that starvation inhibiting active Na+ export. This design of transport module is shown in Figure 1.3.1. Therefore, the functional expression of halorhodopsin and the depletion of ATP reserve in cyanobacteria could be regarded as the keys to the success of biodesalination.
Transformation
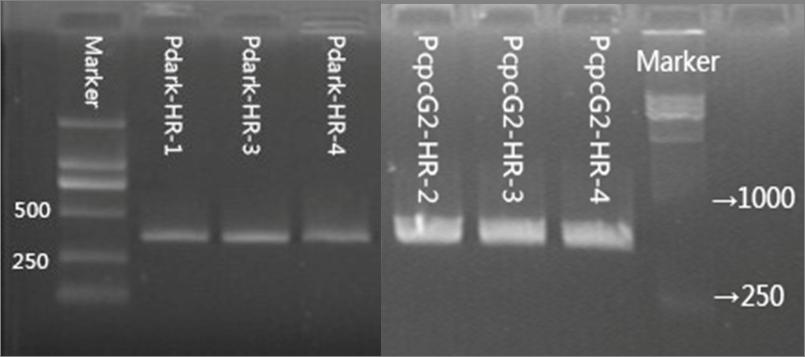
We selected two promoters, PcpcG2 and Pdark, to control the expression of halorhodopsin in Synechosystis sp. strain PCC 6803, which correspond to two composite parts (BBa_K1642010, BBa_K1642011) respectively. We applied kanamycin to screen the transformants and test the transformation by colony PCR. The results of colony PCR are shown in Figure 1.3.2.
Figure 1.3.2 Colony PCR of PcpcG2-HR and Pdark-HR.
The primers for the colony PCR of Pdark-HR and PcpcG2-HR are the same. The forward primer is located in the upstream homologous arm and the reverse one is located in HR.(see detailed construction in Chapter “Composite Parts”) The target fragments for colony PCR of Pdark-HR and PcpcG2-HR are approximately 370bp and 570bp respectively. The number at the tail of the sample represents our number for the colonies of transformant.
Expression
Western Blotting
Western blotting result of Pdark-HR with WT as the control shown in Figure 3 can clearly prove the expression of halorhodpsin in our transformed strain Pdark-HR. Western blotting result of induced culture of PcpcG2-HR compared with non-induced culture shown in Figure 4 can confirm the control mechanism of PcpcG2 and prove the expression of halorhodopsin.
The profile of halorhodopsin on the gel, which consists of three fractions , had been reported by other researchers (Spudich, E. N., & Spudich, J. L. 1985).The reason behind it can be seen in this paper.
Figure 1.3.3 Western blotting result of the transformed strain Pdark-HR with wild type as the negative control.
The induction time is 12h.The molecular weight of halorhodopsin is about 33 kD and the fraction met our expectation.
Figure 1.3.4 Western blotting result of the transformed strain PcpcG2-HR with wild type as the negative control.
Non-induced PcpcG2-HR sample has significant difference with induced PcpcG2-HR.
Immunocytochemistry
We did immunocytochemistry experiments to test the localization of this membrane-integral protein.
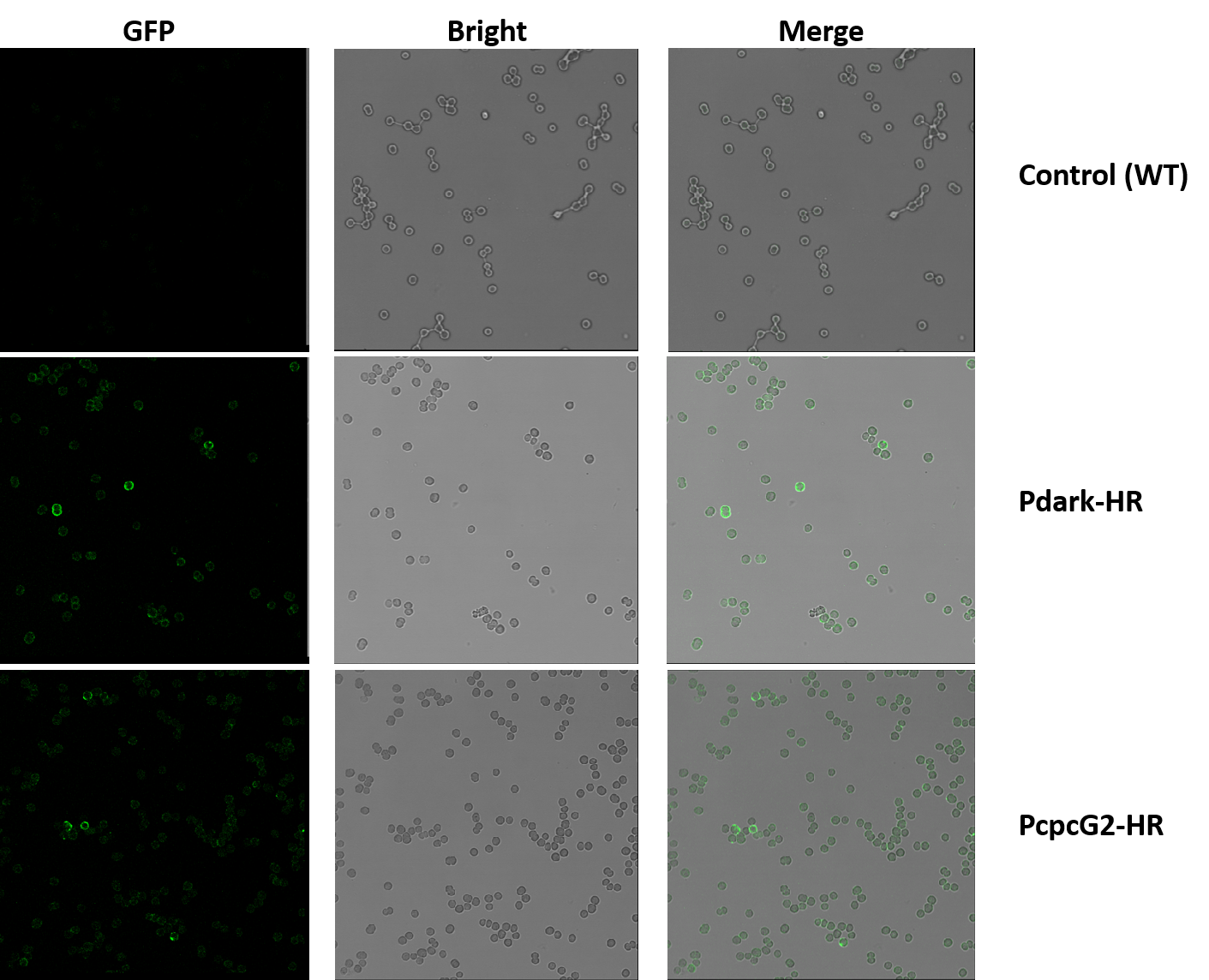
Figure 1.3.5 Detection of Membrane Localization of Halorhodopsin by Immunocytochemistry.
The micrographs are shot by confocal microscope Leica SP5. Line1: control group (wildtype). Line2: experiment group1 (Pdark-HR, BBa_K1642011). Line3: experiment group2 (PcpcG2-HR, BBa_K1642010). Row1: shot under GFP field. Row2: shot under bright field. Row3: the merged micrographs.
The HR is expressed with a His-tag on the C' terminal, thus anti-His mAb is used as the primary antibody and goat anti-mouse IgG Alexa Fluor 488(green fluorescence) as the second antibody.In the experiment groups (Pdark-HR and PcpcG2-HR), many loops of green fluorescence can be seen while the control groups (wildtype) cannot as shown in Figure 2, which indicates that the halorhodopsin expressed successfully on the cell membrane of the genetic cyanobacteria.
References
Amezaga, J. M., Amtmann, A., Biggs, C. A., Bond, T., Gandy, C. J., Honsbein, A., & Templeton, M. R. (2014). Biodesalination: a case study for applications of photosynthetic bacteria in water treatment. Plant physiology, 164(4), 1661-1676.
Spudich, E. N., & Spudich, J. L. (1985). Biochemical characterization of halorhodopsin in native membranes. Journal of Biological Chemistry, 260(2), 1208-1212.
Next: Desalination Process