Difference between revisions of "Team:BGU Israel/Notebook"
| Line 3,245: | Line 3,245: | ||
Life Technologies, 1:250 dilution) was used for visualization. Negative control | Life Technologies, 1:250 dilution) was used for visualization. Negative control | ||
included samples w/o primary Ab. The cells were observed under laser scanning confocal | included samples w/o primary Ab. The cells were observed under laser scanning confocal | ||
| − | microscope (C1si, Nikon). </li> | + | microscope (C1si, Nikon).<a href="https://2015.igem.org/Team:BGU_Israel/Collaborations#Stockholm" target="blank"> See results here</a> </li> |
</ul> | </ul> | ||
</br> | </br> | ||
Latest revision as of 18:11, 18 September 2015

|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
All protocols of our work can be found on our Protocols page .
21.06.15
In the lab today: Shai and Shoham
- We received from Addgene 2 plasmids (in bacterial stab): dCas9-vp64 (#47107) and SaCas9 (#61591). they were spread on LB agar plates with ampicillin ON at 37°C.
- We transformed pSB1C3 backbone into DH5α E. coli and seeded it on LB agar plates with Chloramphenicol.
22.06.15
In the lab today: Shai and Shoham
- 10ml of LB (+ampicillin) was inoculated with one colony from dCas9-vp64 LB agar plate.
- The same was done for SaCas9.
28.06.15
In the lab today: Shai and Shoham
- 10ml of LB (+ampicillin) was inoculated with one colony from dCas9-vp64 LB agar plate.
- The same was done for SaCas9.
29.06.15
In the lab today: Shai
- 10ml of LB (+Chloramphenicol) was inoculated with one colony from pSB1C3 LB agar plate.
- We performed miniprep for dCas9-vp64 and SaCas9.
30.06.15
In the lab today: Shai and Shoham
- SaCas9 (DH5α) glycerol stock was prepared.
- dCas9-vp64 (DH5α) glycerol stock was prepared
- We performed miniprep for pSB1C3.
01.07.15
In the lab today: Shai and Shoham
- Restriction of pSB1C3 backbone with EcoRI and PstI. Alkaline phosphatase was added. Incubation for 30 minutes at 37°C.
- Received U6-gMLP,gMLP,U6-gUBB,gUBB from synthesis in a form of DNA fragments.
- Ligation of pSB1C3 (vector) with U6-gMLP,gMLP,U6-gUBB,gUBB (insert). (each time with different insert).
- 2X10ml of LB (+Chloramphenicol) was inoculated with one colony from pSB1C3 LB agar plate.
We prepared 1% Agarose gel, and run the restriction products.
We extracted the appropriate DNA band (according to size) and performed gel extraction protocol. Nanodrop concentration results were 60 ng/μl.
Restriction of U6-gMLP,gMLP,U6-gUBB,gUBB with EcoRI and PstI. Incubation for 45 minutes at 37°C.
The restriction products were purified using PCR purification kit. Nanodrop concentration results were 3ng/μl.
06.07.15
In the lab today: Shoham
- Ligation products from 01.07 (U6-gMLP-pSB1C3,U6-gUBB-pSB1C3,gMLP-pSB1C3,gUBB-pSB1C3) were transformed into DH5α and seeded on LB agar plates with Chloramphenicol ON.
- Received virus kit from Agilent (cat. No. 240071) (the kit includes helper plasmids, RC plasmids, AAV-MCS plasmids, HEK cells for virus production).
- We transformed pAAV-MCS into DH5α and seeded it on LB agar plates with ampicillin.
07.07.15
In the lab today: Shoham
- No colonies found on any of the plates of 01.07 ligations.
- Colonies found on pAAV-MCS plates from yesterday. 10ml of LB (+ampicillin) was inoculated with one colony.
08.07.15
In the lab today: Shoham
- We performed miniprep for pAAV-MCS. Nanodrop concentration results were 110ng/μl and 117ng/μl (2 Eppendorfs).
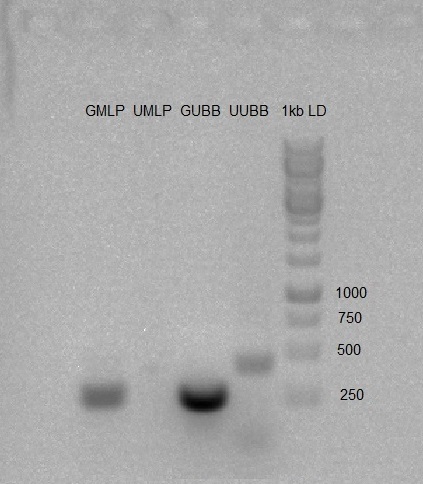
- PCR was carried out on gMLP, gUBB, U6-gMLP, U6-gUBB using default PCR program.
The PCR products were purified using PCR purification kit.
Nanodrop concentration results:
gMLP : 96.6ng/μl, gUBB: 40.5ng/μl, U6-gMLP: 38.0ng/μl, U6-gUBB: 26.0ng/μl.
We prepared 1% Agarose gel, and run the PCR products to check whether the PCR worked as expected. Gel results were good (band in expected size).
Restriction of PCR products with EcoRI and PstI. Incubation for 35 minutes at 37°C.
The restriction products were purified using PCR purification kit. Nanodrop concentration results:
gMLP : 18.8ng/μl, gUBB: 6.1ng/μl, U6-gMLP: 6.7ng/μl, U6-gUBB: 9.4ng/μl.
12.07.15
In the lab today: Shai and Shoham
- Restriction of pSB1C3 backbone with EcoRI and PstI. Alkaline phosphatase was added. Incubation for 30 minutes at 37°C.
- Ligation of pSB1C3 (vector) with U6-gMLP from 08.07(insert).
- Ligation of pSB1C3 (vector) with U6-gUBB from 08.07 (insert).
- Ligation products (U6-gMLP-pSB1C3, U6-gUBB-pSB1C3) were transformed into DH5α and seeded on LB agar plates with Chloramphenicol ON.
- We received from synthesis by Syntezza Bioscience Ltd. hTERT promoter (in pU257 plasmid). It was spread on LB agar plates with ampicillin ON at 37°C.
We prepared 1% Agarose gel, and run the restriction products.
We extracted the appropriate DNA band (according to size) and performed gel extraction protocol.
13.07.15
In the lab today: Shai and Shoham
- PCR was carried out again for gMLP, gUBB, U6-gMLP, U6-gUBB using default PCR program.
- 10ml of LB (+ampicillin) was inoculated with one colony of pAAV-MCS plate from 07.07.
- seeding the following cell lines in 24-well plate (50,000 cells per well in triplicates):
- HepG2 (Hepatocellular carcinoma, ATCC)
- A549 (Lung carcinoma, ATCC)
- HT1080 (Fibrosarcoma, Agilent)
- MDA-MB 231 (Breast cancer cells, ATCC)
- Human dermal fibroblasts (HF)- normal fibroblasts (control)
The PCR products were purified using PCR purification kit.
Nanodrop concentration results:
gMLP : 106.7ng/μl, gUBB: 249.8ng/μl, U6-gMLP: 110.1ng/μl, U6-gUBB: 57.6ng/μl.
Since concentrations of gMLP, U6-gMLP, U6-gUBB are low we tried to run them in purification columns again with 10μl of elution buffer.
New nanodrop concentration results were:
gMLP : 97.2ng/μl, U6-gMLP: 47.4ng/μl, U6-gUBB: 73.1ng/μl.
Cell studies:
14.07.15
In the lab today: Shai, Shoham and Vlad
- We prepared 1% Agarose gel, and run the PCR products from yesterday to check whether the PCR worked as expected. Gel results indicate PCR was fine.
- We performed miniprep for pAAV-MCS from yesterday. Nanodrop concentration results were 169.2ng/μl.
- Colonies found on phTERT plates from 12.07. 10ml of LB (+ampicillin) was inoculated with one colony.
- qPCR for gene expression of hTERT and Survivin (BIRC5)- total RNA was isolated using the EZ-RNA RNA purification kit (Biological Industries), and 500 ng of RNA from each sample was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Life Technologies). For gene expression analysis, mRNA levels were determined by real-time PCR using StepOnePlus™ Applied detection system (Life Technologies) according to the manufacturer's instructions. Gene expression analyses of hTERT and Survivin (BIRC5) were performed using TaqMan gene expression assays (Life Technologies). Data was analyzed by the delta delta Ct method using ACTB as the house-keeping gene.
Cell studies:
15.07.15
In the lab today: Shai, Shoham, Vlad and Bar
- Restriction of PCR products from 13.07 (gMLP, gUBB, U6-gMLP, U6-gUBB) with EcoRI and PstI. Incubation for 35 minutes at 37°C.
- We performed miniprep for phTERT from yesterday. Then restrictions with EcoRI and PstI. Restrictions done in incubation for 30 minutes at 37°C.
We prepared 1% Agarose gel, and run the restriction products of gUBB and U6-gUBB.
We extracted the appropriate DNA band (according to size) and performed gel extraction protocol. Because of human error, U6-gUBB extraction failed. Nanodrop concentration of gUBB: 26.1ng/μl (1.83/2.38).
gMLP and U6-gMLP restriction products were purified using PCR purification kit (so we can compare if one of the purification methods gives better results than the other). Nanodrop concentration results:
gMLP : 31.7ng/μl (1.75/1.55), U6-gMLP: 12.5ng/μl (2.24/1.01).
We run restriction products on the gel prepared earlier today, extracted the appropriate DNA band (according to size) and performed gel extraction protocol.
16.07.15
In the lab today: Shai, Shoham
- Restriction of pSB1C3 backbone with EcoRI and PstI. Alkaline phosphatase was added. Incubation for 30 minutes at 37°C.
- Ligation of pSB1C3 (vector) with phTERT from yesterday (insert).
- Ligation of pSB1C3 (vector) with gMLP from yesterday (insert).
- Ligation of pSB1C3 (vector) with U6-gMLP from yesterday (insert).
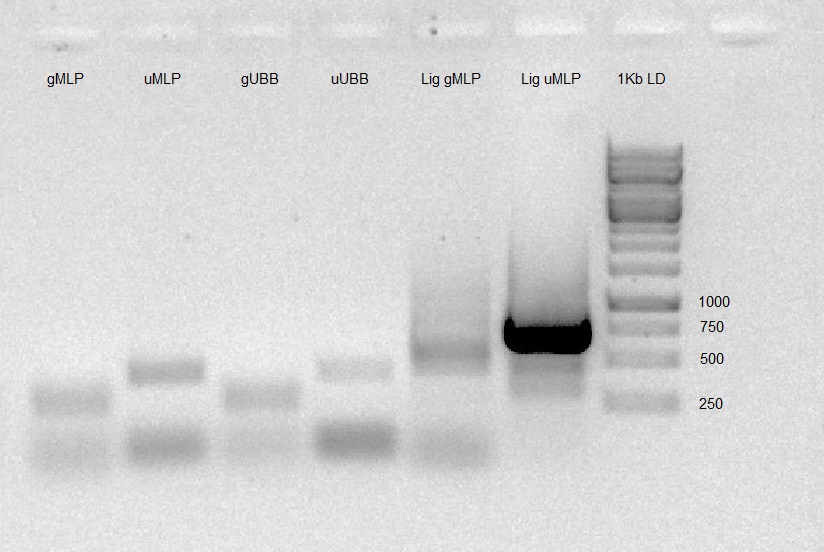
- PCR was carried out on ligation products (gMLP-pSB1C3, U6-gMLP-pSB1C3) to check whether the ligation worked as expected (using F primer of pSB1C3 and R primer of insert). We prepared 1% Agarose gel, and run the PCR products. Gel results were good (band in expected size) so ligation products were transformed into DH5α and seeded on LB agar plates with Chloramphenicol ON.
We prepared 1% Agarose gel, and run the restriction products.
We extracted the appropriate DNA band (according to size) and performed gel extraction protocol.
Ligation products (phTERT-pSB1C3) were transformed into DH5α and seeded on LB agar plates with Chloramphenicol ON.
17.07.15
In the lab today: Shoham
- No colonies found on any of the plates from yesterday.
18.07.15
In the lab today: Shai
- 10ml of LB (+Chloramphenicol) was inoculated with one colony from pSB1C3 LB agar plate.
19.07.15
In the lab today: Shai, Shoham
- Ligation products of gMLP-pSB1C3, U6-gMLP-pSB1C3 from 16.07 were transformed again into DH5 α and seeded on LB agar plates with Chloramphenicol ON.
- We performed miniprep for pSB1C3 from yesterday. Nanodrop concentration results were 85.2ng/μl (2.04,1.84).
- PCR was carried out on gMLP, gUBB, U6-gMLP, U6-gUBB using default PCR program (except Tm was changed form 60°C to 65°C).
The PCR products were purified using PCR purification kit.
Nanodrop concentration results:
gMLP : 100.1ng/μl (1.79,0.72), U6-gMLP: 139.8ng/μl (1.81,1.78),
gUBB : 81.1ng/μl (1.80,1.08), U6-gUBB: 78.9ng/μl (1.83,0.60).
20.07.15
In the lab today: Shai, Shoham, Bar, Vlad
- Colonies found on gMLP-pSB1C3, U6-gMLP-pSB1C3 plates from yesterday.
- Restriction of phTERT with EcoRI and PstI. Restriction done in incubation for 30 minutes at 37°C. Restriction products ran on gel made earlier today.
Colony PCR was carried out on 2 colonies of gMLP-pSB1C3 and 3 colonies of U6-gMLP-pSB1C3 to check whether the ligation worked as expected (using F primer of pSB1C3 and R primer of insert). We prepared 1% Agarose gel, and run the PCR products. Gel results were good (band in expected size).
3X10ml of LB (+Chloramphenicol) was inoculated with 1 colony each of U6-gMLP-pSB1C3 and the same was done for the two colonies of gMLP-pSB1C3.
We extracted the appropriate DNA band (according to size) and performed gel extraction protocol. Nanodrop concentration results were 6.9ng/μl.
21.07.15
In the lab today: Shai, Shoham, Vlad, Shalev
- We performed miniprep for gMLP-pSB1C3, U6-gMLP-pSB1C3 from yesterday. Nanodrop concentration results were:
- Restrictions of gMLP-pSB1C3 and U6-gMLP-pSB1C3 with XhoI (to check ligation). All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel.
- 10ml of LB (+Chloramphenicol) was inoculated with one colony from pSB1C3 LB agar plate.
- 10ml of LB (+ampicillin) was inoculated with one colony from phTERT LB agar plate.
gMLP-pSB1C3 (colony no. 1) 123.4ng/μl.
gMLP-pSB1C3 (colony no. 2) 117.0ng/μl.
U6-gMLP-pSB1C3 (colony no. 1) 103.0ng/μl.
U6-gMLP-pSB1C3 (colony no. 2) 95.0ng/μl.
U6-gMLP-pSB1C3 (colony no. 3) 110.0ng/μl.
Gel results indicate ligation of gMLP-pSB1C3 and U6-gMLP-pSB1C3 succeeded - Send for sequencing.
22.07.15
In the lab today: Shai, Shoham, Vlad, Shalev
- Restrictions of pSB1C3 and phTERT from yesterday with EcoRI and PstI. Restrictions done in incubation for 30 minutes at 37°C. Alkaline phosphatase was added to pSB1C3.
- Ligation of pSB1C3 (vector) with U6-gUBB from 19.07(insert).
- Ligation of pSB1C3 (vector) with gUBB from 19.07 (insert).
- Ligation products (phTERT-pSB1C3,U6-gUBB-pSB1C3, gUBB-pSB1C3) were transformed into DH5 α and seeded on LB agar plates with Chloramphenicol ON.
We prepared 1% Agarose gel, and run the restriction products.
We extracted the appropriate DNA band (according to size) and performed gel extraction protocol.
Ligation of pSB1C3 (vector) with phTERT from (insert).
23.07.15
In the lab today: Shai and Shoham
- Colonies found on all plates from yesterday (phTERT-pSB1C3,U6-gUBB-pSB1C3, gUBB-pSB1C3). 4 colonies were taken from each plate and 12X10ml of LB (+Chloramphenicol) was inoculated with one colony each.
24.07.15
In the lab today: Shai and Shoham
- We performed miniprep for all phTERT-pSB1C3,U6-gUBB-pSB1C3, gUBB-pSB1C3 from yesterday. Nanodrop concentration checked for all:
- Restrictions of miniprep products with EcoRI and PstI (to check ligation). All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel.
|
phTERT-pSB1C3 (ng/μl) |
U6-gUBB-pSB1C3 (ng/μl) |
gUBB-pSB1C3 (ng/μl) |
|
|
LB no. 1 |
233 |
- |
231 |
|
LB no. 2 |
309 |
249 |
154 |
|
LB no. 3 |
199 |
227 |
123 |
|
LB no. 4 |
248 |
246 |
187 |
Gel results indicate ligation of phTERT-pSB1C3,U6-gUBB-pSB1C3, gUBB-pSB1C3 succeeded - Send for sequencing of 2 colonies from each.
26.07.15
In the lab today: Shoham
- 10ml of LB (+ampicillin) was inoculated with one colony from dCas9-vp64 LB agar plate.
- The same was done for SaCas9.
27.07.15
In the lab today: Shai and Shoham
- We performed miniprep for SaCas9, dCas9-vp64 from yesterday (2 Eppendorfs each). Nanodrop concentration results were:
- We received pSurvivin-mCherry from outsourcing synthesis by Syntezza Bioscience Ltd.
- We transformed plasmids from virus kit (pAAV-MCS, pRC, pHelper) and pSurvivin-mCherry into DH5α and seeded them on LB agar plates with ampicillin.
|
SaCas9 |
dCas9-vp64 |
|
|
Eppendorf no. 1 |
277ng/μl (2.01/2.06) |
593ng/μl (1.85/2.09) |
|
Eppendorf no. 2 |
215ng/μl (1.98/2.09) |
522ng/μl (1.89/2.30) |
28.07.15
In the lab today: Shai and Shoham
- Colonies found on all plates from yesterday. 2 colonies were taken from each plate and 8X10ml of LB (+ampicillin) was inoculated with one colony each.
29.07.15
In the lab today: Vlad and Shalev
- We performed miniprep for pAAV-MCS, pRC, pHelper, pSurvivin-mCherry from yesterday (2 Eppendorfs each). Nanodrop concentration results were:
|
colony no. 1 |
colony no. 2 |
|
|
pHelper |
602ng/μl |
236ng/μl |
|
pRC |
520ng/μl |
399ng/μl |
|
pAAV-MCS |
186ng/μl |
137ng/μl |
|
pSurvivin-mCherry |
305ng/μl |
357ng/μl |
02.08.15
In the lab today: Shai and Shoham
- We transformed gMLP-pSB1C3 (from colony no. 2 at 21.07) into DH5α E. coli and inserted into 10ml of LB (+Chloramphenicol) ON.
- We transformed U6-gMLP-pSB1C3 (from colony no. 3 at 21.07) into DH5α E. coli and seeded it on LB agar plates with Chloramphenicol.
- 10ml of LB (+Chloramphenicol) was inoculated with colony no. 3 of phTERT-pSB1C3 from 23.07.
- 10ml of LB (+Chloramphenicol) was inoculated with colony no. 2 of U6-gUBB-pSB1C3 from 23.07.
- We transformed pGFPN1 (GFP plasmid found on our lab) into DH5α and seeded it on LB agar plates with ampicillin.
03.08.15
In the lab today: Shai and Shoham
- Nothing grew in LB of gMLP-pSB1C3 and U6-gMLP-pSB1C3 from yesterday, so we did transformation again, this time with heat shock:
- Colonies found on pGFPN1 plate from yesterday. 10ml of LB (+ampicillin) was inoculated with one colony.
gMLP-pSB1C3 (colony no. 2 from 21.07), U6-gMLP-pSB1C3 (colony no. 2 from 21.07) and U6-gMLP-pSB1C3 (colony no. 3 from 21.07) all were inserted into 10ml of LB (+Chloramphenicol) ON.
04.08.15
In the lab today: Vlad
- We performed miniprep for: gMLP-pSB1C3 (colony no. 2), U6-gMLP-pSB1C3 (colony no. 2), U6-gMLP-pSB1C3 (colony no. 3), pGFPN1 (all from yesterday) and phTERT-pSB1C3 from 02.08. Nanodrop concentration results were:
- Restriction of pAAV-MCS and pGFPN1 with EcoRI and XbaI. Alkaline phosphatase was added to pAAV-MCS. Incubation for 30 minutes at 37°C.
gMLP-pSB1C3(2): 245ng/μl, U6-gMLP-pSB1C3(2): 221ng/μl,
U6-gMLP-pSB1C3(3): 256ng/μl, phTERT-pSB1C3(3): 58.6ng/μl, pGFPN1: 2.4ng/μl.
We prepared 1% Agarose gel, and run the restriction products.
Gel results indicate that restriction of pGFPN1 failed.
05.08.15
In the lab today: Shai
- We performed miniprep for: U6-gUBB-pSB1C3 (colony no. 2) from 02.08. Nanodrop concentration results were 160ng/μl.
- Send for sequencing miniprep results of yesterday and today (phTERT-pSB1C3(3), gMLP-pSB1C3(2), U6-gMLP-pSB1C3 (2), U6-gMLP-pSB1C3(3), U6-gUBB-pSB1C3(2)).
06.08.15
In the lab today: Shai, Vlad
- We decided to try another GFP plasmid (instead of pGFPN1). New plasmid: pAM-EGFP.
- Restriction of pAAV-MCS and pAM-EGFP with HindIII and BamHI. Alkaline phosphatase was added to pAAV. Incubation for 30 minutes at 37°C.
- Ligation of pAAV (vector) with GFP (insert).
We prepared 1% Agarose gel, and run the restriction products.
We extracted the appropriate DNA bands (according to size) and performed gel extraction protocol. Nanodrop concentration results were
GFP : 19.6ng/μl and pAAV: 9.7ng/μl.
Ligation products (GFP-pAAV) were transformed into DH5α and seeded on LB agar plates with ampicillin ON.
09.08.15
In the lab today: Shai
- No colonies found on GFP-pAAV plates from yesterday, so we did restriction again.
- Ligation of pAAV (vector) with GFP (insert).
- 2X100ml of LB (+ampicillin) were made for midiprep tomorrow. One was inoculated with a colony of pHelper and the other with pRC.
Restriction of pAAV-MCS and pAM-EGFP with HindIII and BamHI. Alkaline phosphatase was added to pAAV. Incubation for 30 minutes at 37°C.
We prepared 1% Agarose gel, and run the restriction products.
We extracted the appropriate DNA bands (according to size) and performed gel extraction protocol.
Ligation products (GFP-pAAV) were transformed into DH5α and seeded on LB agar plates with ampicillin ON.
10.08.15
In the lab today: Shai , Shoham
- 2 colonies found on GFP-pAAV plates from yesterday. 2X10ml of LB (+ampicillin) was inoculated with one colony each.
- We performed midiprep for pHelper, pRC, pAAV-MCS. Nanodrop concentration results were:
|
Vessel no. 1 |
Vessel no. 2 |
|
|
pRC |
600ng/μl (1.82/2.22) |
646ng/μl (1.84/2.13) |
|
pHelper |
658ng/μl (1.87/2.24) |
581ng/μl |
|
pAAV-MCS |
217ng/μl (1.80/1.53) |
277ng/μl (1.79/1.56) |
11.08.15
In the lab today: Vlad , Shalev
- We received phTERT-eGFP-pAAV plasmid (master-AAV) from IDT. It was transformed into DH5α and inserted into 10ml of LB (+ampicillin) ON.
- We performed miniprep for GFP-pAAV from yesterday.
- Restriction of GFP-pAAV with HindIII and BamHI (to check ligation). All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel.
Nanodrop concentration results were:
colony no. 1: 189.6ng/μl (1.96,2.05), colony no. 2: 182.6ng/μl (1.94,2.03).
Results indicate ligation of GFP-pAAV in colony no. 2 succeeded.
12.08.15
In the lab today: Shoham, Vlad and Shalev
- master-AAV (DH5α) glycerol stock was prepared
- GFP-AAV (DH5α) glycerol stock was prepared
- PCR was carried out on U6-gMLP, U6-gUBB, Affibody using default PCR program.
- We performed midiprep for master-AAV. Nanodrop concentration results were 286.8ng/μl (1.87/2.28).
- Restriction of U6-gMLP, U6-gUBB with SalI and PacI. Incubation for 30 minutes at 37°C.
The PCR products were purified using PCR purification kit.
Nanodrop concentration results of U6-gMLP were 160.2ng/μl (1.84/1.92).
Nanodrop concentration results of U6-gUBB were 219.8ng/μl (1.85/1.08).
Nanodrop concentration results of Affibody were 109.0ng/μl (1.84/2.11).
We prepared 1% Agarose gel, and run the PCR products.
13.08.15
In the lab today: Shoham, Vlad, Bar and Shalev
- Restriction of DNA . All restrictions done in incubation for 30 minutes at 37°C. Then we run restriction products on 1% Agarose gel, extracted the appropriate DNA band (according to size) and performed gel extraction protocol. Nanodrop concentration checked.
- Ligations :
- Ligation of pAAV (vector) with Affibody (insert).
- Ligation of master-AAV (vector) with U6-gMLP (insert).
- Ligation of master-AAV (vector) with U6-gUBB (insert)
- Ligation of master-AAV (vector) with dCas9-vp64 (insert).
- Ligation of master-AAV (vector) with SaCas9 (insert).
|
Restriction enzymes |
Alkaline phosphatase |
Nanodrop concentration (ng/μl) |
|
|
pAAV_MCS |
EcoRI, XhoI |
Added |
50 |
|
master-AAV (1) |
SalI, PacI |
Added |
56 |
|
master-AAV (2) |
SalI, XbaI |
Added |
74 |
|
master-AAV (3) |
XhoI, BamHI |
Added |
64 |
|
pSurvivin-mCherry |
SalI, PacI |
13.2 |
|
|
dCas9-vp64 |
SalI, XbaI |
43 |
|
|
SaCas9 |
XhoI, BamHI |
29 |
|
|
Affibody * |
EcoRI, XhoI |
50 |
*Affibody was purified using PCR purification kit (not gel extraction).
After ligation, products were transformed into DH5α and seeded on LB agar plates with ampicillin ON.
15.08.15
In the lab today: Shoham
- No colonies found on any of the plates from yesterday. So we do all restrictions and ligation again.
- Restriction of DNA. All restrictions done in incubation for 30 minutes at 37°C. Then we run restriction products on 1% Agarose gel, extracted the appropriate DNA band (according to size) and performed gel extraction protocol. Since it is Saturday nanodrop room is closed so we didn't check the concentration and didn't continue to ligation (nanodrop results added later).
- Since we can't do ligation with current products of restriction, we tried ligation of yesterday's product again:
- Ligation of pAAV (vector) with Affibody (insert).
- Ligation of master-AAV (vector) with U6-gMLP (insert).
- Ligation of master-AAV (vector) with U6-gUBB (insert)
- Ligation of master-AAV (vector) with dCas9-vp64 (insert).
- Ligation of master-AAV (vector) with SaCas9 (insert).
- Ligation of master-AAV (vector) with pSurvivin-mCherry (insert).
|
Restriction enzymes |
Alkaline phosphatase |
Nanodrop results - eppendorf 1 |
Nanodrop results - eppendorf 2 |
|
|
pAAV_MCS (from stock) |
EcoRI, XhoI |
Added |
- |
- |
|
master-AAV (1) |
SalI, PacI |
Added |
78.7 (2.47/0.55) |
119.0(1.87/0.69) |
|
master-AAV (2) |
SalI, XbaI |
Added |
74.5 (2.51/0.51) |
68.0 (1.85/1.43) |
|
master-AAV (3) |
XhoI, BamHI |
Added |
72.9 (2.19/0.29) |
56.9 (1.94/1.04) |
|
pSurvivin-mCherry |
SalI, PacI |
34.2 (2.37/0.06) |
11.1 (1.79/0.13) |
|
|
dCas9-vp64 |
SalI, XbaI |
43.3 (2.78/0.18) |
30.3 (2.06/1.41) |
|
|
SaCas9 (from stock) |
XhoI, BamHI |
30.0 |
20.0 |
|
|
U6-gUBB |
SalI, PacI |
25.6 (5.26/0.21) |
14.0 (1.67/0.30) |
|
|
U6-gMLP |
SalI, PacI |
21.7 (4.59/0.06) |
12.8 (1.71/0.08) |
|
|
Affibody * |
EcoRI, XhoI |
17.0 |
- |
*Affibody was purified using PCR purification kit (not gel extraction).
After ligation, products were transformed into DH5α and seeded on LB agar plates with ampicillin ON.
16.08.15
In the lab today: Shoham, Vlad
- Colonies found on pSurvivin-mCherry-pAAV plates from yesterday. 10ml of LB (+ampicillin) was inoculated with one colony.
- Colonies found on CMV-dCas9-VP64-pAAV plates from yesterday. 2X10ml of LB (+ampicillin) was inoculated with one colony each.
- Colonies found on Affibody-AAV plates from yesterday. 10ml of LB (+ampicillin) was inoculated with one colony.
- No colonies found on the other plates from yesterday. So we do ligation with yesterday's restriction products:
- Ligation of master-AAV (vector) with U6-gMLP (insert).
- Ligation of master-AAV (vector) with U6-gUBB (insert)
- Ligation of master-AAV (vector) with SaCas9 (insert).
17.08.15
In the lab today: Vlad, Shalev
- Nothing grew in LB of pSurvivin-mCherry-pAAV from yesterday.
- LB of CMV-dCas9-VP64-pAAV and LB of Affibody-AAV are OK. Miniprep was performed on both. Nanodrop concentration results were 193.5ng/μl (1.95/20.9) for CMV-dCas9-VP64-pAAV and 209.7ng/μl (1.97/2.09) for pAffibody-AAV.
- Yesterday's ligation products (U6-gMLP-pAAV, U6-gUBB-pAAV, CMV-SaCas9-pAAV) were transformed into DH5α and seeded on LB agar plates with ampicillin ON.
- Ligation products of CMV-dCas9-VP64-pAAV (from 15.08) were transformed again into DH5α and seeded on LB agar plates with ampicillin ON.
We prepared 1% Agarose gel, and run Miniprep products.
Gel results indicate that ligation of CMV-dCas9-VP64-pAAV failed.
Ligation of pAffibody-AAV succeeded.
Affibody-AAV (DH5α) glycerol stock was prepared.
18.08.15
In the lab today: Shoham, Vlad, Shalev
- Restrictions again of master-AAV, pSurvivin-mCherry, U6-gUBB, U6-gMLP with SalI, PacI. All restrictions done in incubation for 30 minutes at 37°C. Alkaline phosphatase was added to master-AAV.
- Restriction products of master-AAV, pSurvivin-mCherry ran on 1% Agarose gel.
- The restriction products of U6-gUBB, U6-gMLP were purified using PCR purification kit. Nanodrop concentration results were 21.1ng/μl for U6-gUBB and 35.9ng/μl for U6-gMLP.
- We inoculated 10ml of LB (+ampicillin) with one colony from CMV-SaCas9-pAAV from yesterday.
- We inoculated 10ml of LB (+ampicillin) with one colony from pSurvivin-mCherry-pAAV from 16.08.
Gel results indicate about a problem. Tomorrow we will use new master-AAV from midiprep and new pSurvivin-mCherry from glycerol stock (10ml of LB (+ampicillin) was inoculated).
19.08.15
In the lab today: Vlad, Shalev
- Nothing grew in LB of pSurvivin-mCherry-pAAV from yesterday.
- LB of CMV-SaCas9-pAAV is OK. Miniprep was performed.
- We performed midiprep for master-AAV and miniprep for pSurvivin-mCherry. Then restrictions of both with SalI, PacI. All restrictions done in incubation for 30 minutes at 37°C. Alkaline phosphatase was added to master-AAV.
- Ligations :
- Ligation of master-AAV (vector) with pSurvivin-mCherry (insert). (Survivin from today).
We prepared 1% Agarose gel, and ran Miniprep products.
Gel results indicate that ligation of CMV-SaCas9-pAAV failed.
We run restriction products on the gel prepared earlier today, extracted the appropriate DNA band (according to size) and performed gel extraction protocol.
Ligation of master-AAV (vector) with pSurvivin-mCherry (insert). (Survivin from 16.08).
Ligation of master-AAV (vector) with pSurvivin-mCherry (insert). (Survivin from 13.08).
Ligation of master-AAV (vector) with U6-gMLP (insert). (U6-gMLP from 18.08).
Ligation of master-AAV (vector) with U6-gUBB (insert). (U6-gUBB from 18.08).
After ligation, products were transformed into DH5α and seeded on LB agar plates with ampicillin ON.
20.08.15
In the lab today: Vlad, Shalev
- No colonies found on any of the plates from yesterday.
- We think maybe there is a problem with the master-AAV. So we decided to try master-AAV from 2 dates: 19.08 and 16.08 for today's ligations:
Ligation of master-AAV (vector) with pSurvivin-mCherry (insert). (master-AAV from 19.08).
Ligation of master-AAV (vector) with pSurvivin-mCherry (insert). (master-AAV from 16.08).
Ligation of master-AAV (vector) with U6-gUBB (insert). (master-AAV from 19.08).
Ligation of master-AAV (vector) with U6-gMLP (insert). (master-AAV from 16.08).
After ligation, products were transformed into DH5α and seeded on LB agar plates with ampicillin ON.
21.08.15
In the lab today: Shoham, Vlad, Shalev
- No colonies found on any of the plates from yesterday.
- We start working on pRC and pHelper of AAV kit.
Both were transformed into DH5α and inserted into 10ml of LB (+ampicillin) ON.
22.08.15
In the lab today: Shoham
- We performed midiprep for pRC. Nanodrop concentration results were 3745ng/μl (1.81/2.24).
- We performed midiprep for pHelper. Nanodrop concentration results were 4072ng/μl (1.79/2.17).
23.08.15
In the lab today: Vlad, Shalev
- Since no colonies found on any of the plates in 21.08, we do DNA restrictions again.
- Gel result indicates that master-AAV restriction didn't work as expected. Maybe master-AAV eppendorf is contaminated. So master-AAV from original stock was transformed into DH5α and inserted into 10ml of LB (+ampicillin) ON.
- pSurvivin-mCherry gel results are OK.
- Meanwhile, we decided to try ligation with current master-AAV. So both master-AAV and pSurvivin-mCherry extracted from gel.
- Since it didn't work so far, we tried to improvise with the ligation protocol. Instead of 50ng of vector, we took 100ng. Instead of 150ng of insert, we took 400ng. So the vector/insert ratio was changed from 1:3 to 1:4.
- After ligation, products were transformed into DH5α and seeded on LB agar plates with ampicillin ON. We also made a change in transformation protocol - instead of 10 minutes in ice, we left the DH5α for 40 minutes in the ice.
- Seeding six 15 cm tissue culture plates of AAV-293 cells (Agilent) for viral stocks production (2 plates per each vector).
- Seeding 6-well plates of HT1080 and HF cells (200,000 cell/ well) for CaP transfection.
Restriction of master-AAV, pSurvivin-mCherry with SalI and PacI. Incubation for 30 minutes at 37°C. Alkaline phosphatase was added to master-AAV.
We ran restriction products on 1% Agarose gel.
Ligation of master-AAV (vector) with dCas9-vp64 (insert).
Ligation of master-AAV (vector) with SaCas9 (insert).
Cell studies:
24.08.15
In the lab today: Shoham, Vlad, Shalev
- We performed miniprep for master-AAV from yesterday (from stock). Nanodrop concentration result was 233ng/μl.
- Colonies found on CMV-SaCas9-pAAV plate from yesterday. 10ml of LB (+ampicillin) was inoculated with one colony ON
- Ligations:
- AAV plasmids arrived from cloning: phTERT-SaCas9-pAAV, phTERT-dCas9-VP64-pAAV, pMLPm-eGFP-pAAV, pSurvivin-gMLP-pAAV, pSurvivin-gUBB-pAAV.
- Three transfections were performed in AAV-293 cells according to AAV production protocol.
- GFP-pAAV
- master-AAV
- Affibody-AAV
- In addition two transfections were performed according to CaP transfection protocol in HT1080 and HF.
- GFP-pAAV
- master-AAV
Restriction of master-AAV was made with SalI, PacI. Restriction done in incubation for 35 minutes at 37°C. Alkaline phosphatase was added.
Restriction products ran on 1% Agarose gel. master-AAV gel results are good.
2X Ligation of today's master-AAV (vector) with yesterday's pSurvivin-mCherry (insert).
Ligation of yesterday's master-AAV (vector) with U6-gMLP (insert).
After ligation, products were transformed into DH5α and seeded on LB agar plates with ampicillin ON. Like yesterday, for one of pSurvivin-mCherry-pAAV ligations we changed the protocol - instead of 10 minutes in ice, we left the DH5α for 40 minutes in the ice.
Plasmids were transformed into DH5α and inserted into 100ml of LB (+ampicillin) ON, for midiprep tomorrow.
Cell studies:
The transfections were with the following Plasmids:
25.08.15
In the lab today: Vlad, Shalev
- We performed midiprep for pSurvivin-gMLP-pAAV, pSurvivin-gUBB-pAAV, phTERT-dCas9-VP64-pAAV . Nanodrop concentration results were:
- Restrictions of CMV-SaCas9-pAAV with NotI, AgeI (to check ligation). All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel. Gel results indicate ligation of CMV-SaCas9-pAAV failed.
- Colonies found on all plates from yesterday (2XpSurvivin-mCherry-pAAV, 1XU6-gMLP-pAAV). 10ml of LB (+ampicillin) was inoculated with 2 colonies each.
- Colonies found on CMV-SaCas9-pAAV plates from 23.08. 10ml of LB (+ampicillin) was inoculated with one colony.
- Medium change in all plates.
pSurvivin-gMLP-pAAV : 1379ng/μl (1.85/2.25) - concentration is good.
pSurvivin-gUBB-pAAV : 270ng/μl (1.86/2.24) - concentration is too low.
phTERT-dCas9-VP64-pAAV: concentration is too low.
Cell studies:
26.08.15
In the lab today: Vlad, Shalev
- Restrictions of pSurvivin-mCherry-pAAV with SalI, AgeI (to check ligation). All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel.
- Restrictions of U6-gMLP-pAAV with SalI, PstI (to check ligation). All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel.
- Plasmids (pSurvivin-mCherry-pAAV and U6-gMLP-pAAV) were transformed into DH5α and inserted into 100ml of LB (+ampicillin) ON, for midiprep tomorrow.
- AAV Plasmids (phTERT-SaCas9-pAAV, phTERT-dCas9-VP64-pAAV, pMLPm-eGFP-pAAV, pSurvivin-gUBB-pAAV) were transformed into DH5α and inserted into 100ml of LB (+ampicillin) ON, for midiprep tomorrow.
- Ligation of master-AAV from 23.08 (vector) with U6-gUBB (insert).
- Observation: CaP transfection in HT1080 and HF at 48h, we looked at cells under inverted fluorescence microscope (IX70, Olympus) connected to an Olympus (DD71) digital capture system.
- Seeding eight 15 cm tissue culture plates of AAV-293 cells (Agilent) for viral stocks production.
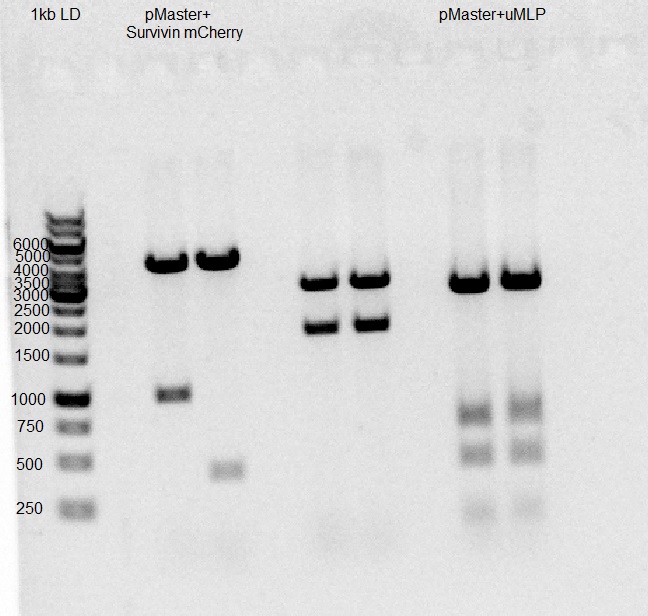
Gel results indicate ligation of pSurvivin-mCherry-pAAV succeeded.
Gel results indicate ligation of U6-gMLP-pAAV succeeded.
After ligation, products were transformed into DH5α and seeded on 2 LB agar plates with ampicillin ON.
Cell studies:
27.08.15
In the lab today: Vlad, Shalev
- We performed midiprep. Nanodrop concentration checked.
- Colonies found on both plates of U6-gUBB-pAAV from yesterday. 5X10ml of LB (+ampicillin) was inoculated with one colony each ON (3 colonies from plate 1, and 2 colonies from plate 2).
- Preparing viral stocks according to AAV production protocol for:
- GFP-pAAV
- master-AAV
- Affibody-AAV
- Four transfections were performed in AAV-293 cells according to AAV production protocol.
- pSurvivin-mCherry-pAAV
- phTERT-dCas9-VP64-pAAV
- pSurvivin-gMLP-pAAV
- pMLPm-eGFP-pAAV
|
Nanodrop results |
|
|
phTERT-SaCas9-pAAV |
893ng/μl (1.86/2.29) |
|
phTERT-dCas9-VP64-pAAV |
688ng/μl (1.84/2.35) |
|
pMLPm-eGFP-pAAV |
956ng/μl (1.86/2.28) |
|
pSurvivin-gUBB-pAAV |
349ng/μl (1.84/2.24) |
|
pSurvivin-mCherry-pAAV |
450ng/μl (1.84/2.25) |
Cell studies:
The transfections were with the following Plasmids:
28.08.15
In the lab today: Vlad, Shalev
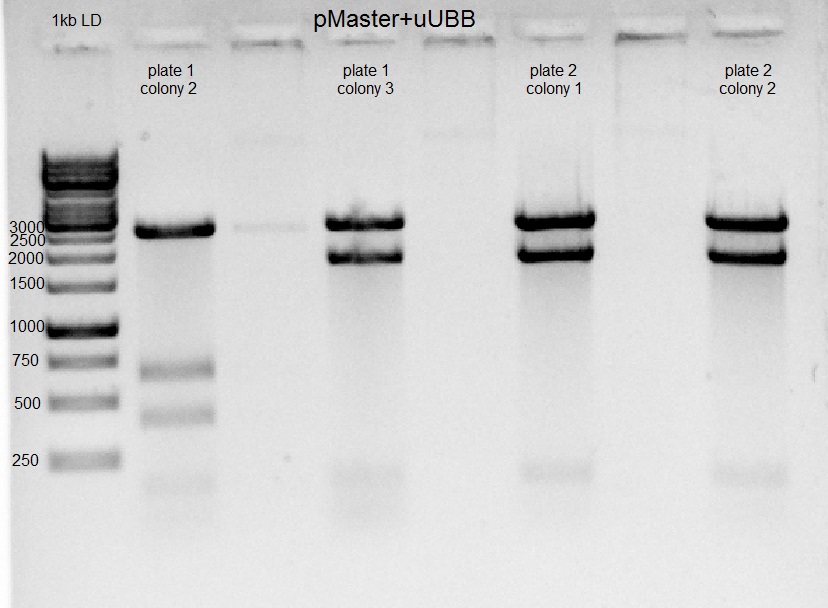
- We performed miniprep for U6-gUBB-pAAV from yesterday
- Restriction of DNA . All restrictions done in incubation for 30 minutes at 37°C. Then we run restriction products on 1% Agarose gel, extracted the appropriate DNA band (according to size) and performed gel extraction protocol.
- Change medium in AAV-293 cell culture plates
Then we perform Restrictions of with SalI, PstI (to check ligation). All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel.
Gel results indicate that in the second colony of the first plate ligation of U6-gUBB-pAAV succeeded.
|
Restriction enzymes |
Alkaline phosphatase |
|
|
master-AAV (1) |
XhoI, BamHI |
Added |
|
master-AAV (2) |
SalI, XbaI |
Added |
|
dCas9-vp64 |
SalI, XbaI |
|
|
SaCas9 |
XhoI, BamHI |
Cell studies:
29.08.15
In the lab today: Vlad, Shalev
- Ligations of restrictions from yesterday:
- U6-gUBB-pAAV (DH5α) glycerol stock was prepared.
Ligation of master-AAV (vector) with dCas9-vp64 (insert).
Ligation of master-AAV (vector) with SaCas9 (insert).
After ligation, products were transformed into DH5α and seeded on LB agar plates with ampicillin ON.
30.08.15
In the lab today: Vlad, Shalev
- Colonies found on both plates (CMV-dCas9-VP64-pAAV and CMV-SaCas9-pAAV) from yesterday. 10ml of LB (+ampicillin) was inoculated with one colony from each ON.
- We still had some restriction products of master-AAV and dCas9-vp64 from 28.08, so we did Ligation of master-AAV (vector) with dCas9-vp64 (insert) again. (in case ligation failed in current colonies). After ligation, products were transformed into DH5α and seeded on LB agar plates with ampicillin ON.
- Preparing viral stocks of according to AAV production protocol
- pSurvivin-mCherry-pAAV
- phTERT-dCas9-VP64-pAAV
- pSurvivin-gMLP-pAAV
- pMLPm-eGFP-pAAV
- Seeding HT1080 and HF cells in 24-well plates (50,000 cells per well), and in 6- well plates (200,000 cells per well)
Cell studies:
31.08.15
In the lab today: Vlad, Shalev
- Colonies found on CMV-dCas9-VP64-pAAV plate from yesterday. 10ml of LB (+ampicillin) was inoculated with one colony ON.
- pSurvivin-mCherry-pAAV (DH5α) glycerol stock was prepared.
- Restrictions of CMV-dCas9-VP64-pAAV with SpeI, PacI (to check ligation). All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel. Gel results indicate about a problem. Maybe PacI enzyme is not working as expected. We will try tomorrow other restriction enzymes set.
- Restrictions of CMV-SaCas9-pAAV with AgeI, NotI (to check ligation). All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel.
- CMV-SaCas9-pAAV (DH5α) glycerol stock was prepared.
- Functional experiment, activation platform: transduction of HT1080 cells and HF cells (24- well plates) with 40 µl from AAV stocks of:
- Three components of activation system: phTERT-dCas9-VP64-pAAV, pSurvivin-gMLP-pAAV, pMLPm-eGFP-pAAV
- Control for transduction efficiency: GFP-pAAV
- Controls for promoters activation: pSurvivin-mCherry-pAAV, phTERT-eGFP-pAAV
- Same groups of AAV vectors were tested in HT1080 and HF (6- well plates) using CaP transfection protocol. In this experiment we included also a group of pMLPm-eGFP-pAAV alone to exclude false positive GFP expression results by activation of the minimal promoter.
Gel results indicate ligation of CMV-SaCas9-pAAV succeeded.
Cell studies:
01.09.15
In the lab today: Vlad, Shalev
- Restrictions of CMV-dCas9-VP64-pAAV from 29.08 and 30.08 (to check ligation). We decided to use different restriction enzymes because maybe PacI is problematic. So new enzymes are: SalI,XBaI. All restrictions done in incubation for 30 minutes at 37°C. Restriction products ran on 1% Agarose gel.
- CMV-dCas9-VP64-pAAV (DH5α) glycerol stock was prepared.
- 100ml of LB (+ampicillin) where made for midiprep. Each contained one of the following: CMV-dCas9-VP64-pAAV, CMV-SaCas9-pAAV, U6-gMLP-pAAV, U6-gUBB-pAAV.
- Change medium to HT1080 and HF cells in 6-well plates
Gel results indicate ligation of CMV-dCas9-VP64-pAAV from 29.08 succeeded.
Cell studies:
02.09.15
In the lab today: Shoham, Shalev, Vlad
- Functional experiment, activation platform - Observation: CaP transfection and AAV transduction in HT1080 and HF at 48h, we looked at cells under inverted fluorescence microscope (IX70, Olympus) connected to an Olympus (DD71) digital capture system. See results here
- Seeding 15 cm tissue culture plates of AAV-293 cells (Agilent) for viral stocks production.
Cell studies:
03.09.15
In the lab today: Vlad, Shalev
- We performed midiprep. Nanodrop concentration checked.
- Six transfections were performed in AAV-293 cells according to AAV production protocol.
- CMV-dCas9-VP64-pAAV
- CMV-SaCas9-pAAV
- U6-gMLP-pAAV
- U6-gUBB-pAAV
- phTERT-SaCas9-pAAV
- pSurvivin-gUBB-pAAV
|
Nanodrop results |
|
|
CMV-dCas9-VP64-pAAV |
1467ng/μl |
|
CMV-SaCas9-pAAV |
697ng/μl |
|
U6-gMLP-pAAV |
1685ng/μl |
|
U6-gUBB-pAAV |
1209ng/μl |
Cell studies:
The transfections were with the following Plasmids:
04.09.15
In the lab today: Shoham, Shalev, Vlad
- Change medium in AAV-293 cell culture plates
Cell studies:
06.09.15
In the lab today: Shoham, Shalev, Vlad
- Seeding HT1080 and HF cells in 24-well plates (50,000 cells per well), and in 6- well plates (200,000 cells per well).
- For affibody exp- seeding HT1080 cells on chamber slides (10,000 cells per well)
Cell studies:
07.09.15
In the lab today: Shoham, Shalev, Shai
- Functional experiment, knockout platform: transduction of HT1080 cells and HF cells (24- well plates) with 40 µl from AAV stocks of:
- Two components of knockout system, specific promoters:
- Two components of knockout system, constitutive promoters:
- Controls: phTERT-eGFP-pAAV, pSurvivin-mCherry-pAAV
- Same groups of AAV vectors were tested in HT1080 and HF (6- well plates) using CaP transfection protocol.
- Transduction of affibody-AAV was performed in HT1080 (chamber slides)
Cell studies:
phTERT-SaCas9-pAAV, pSurvivin-gUBB-pAAV
CMV-SaCas9-pAAV, U6-gUBB-pAAV
08.09.15
In the lab today: Shoham, Shalev, Shai
- Change medium to HT1080 and HF cells in 6-well plates
Cell studies:
10.09.15
In the lab today: Shoham, Shalev, Shai
- Observation: we looked at cells under inverted fluorescence microscope (IX70, Olympus) connected to an Olympus (DD71) digital capture system.
- Immunostaining for Affibody expression according to Immunofluorescent staining protocol: cells in chamber slides were stained with primary Ab for FLAG peptide (F1804, Sigma, 1:500 dilution). Protocols with and w/o permeabilization step were used, to distinguish between external and intracellular expression. Alexa-488-conjugated Ab (A21200, Life Technologies, 1:250 dilution) was used for visualization. Negative control included samples w/o primary Ab. The cells were observed under laser scanning confocal microscope (C1si, Nikon). See results here
Cell studies:















Follow us:
Address:
Ben Gurion 1, Beer Sheva 8410501, Israel
Mail: igembgu2015@gmail.com