Difference between revisions of "Team:Sydney Australia/BusinessModel"
| Line 35: | Line 35: | ||
=Current Situation= | =Current Situation= | ||
| − | Ethylene oxide, one of the most abundantly produced compounds, is derived from ethylene via an oxidation process. The | + | Ethylene oxide, one of the most abundantly produced chemical compounds, is derived from ethylene via an oxidation process. The sectors in which this compound is utilised include (but are not limited to) the pharmaceutical, medical, and manufacturing industries. The world production of ethylene oxide is \(17\times10^6\) metric tons<sup>2</sup> and will only increase on the back of the expanding economies of countries such as China and India. |
| − | Due to the abundant use and versatility of ethylene oxide it is evident this compound is essential to our society: Thus, it is imperative we find a | + | Due to the abundant use and versatility of ethylene oxide it is evident this compound is at present essential to our society: Thus, it is imperative we find a new avenue for production of this critical substance in an environmentally friendly and cost efficient manner. |
| Line 54: | Line 54: | ||
==Current Methods of Production== | ==Current Methods of Production== | ||
| − | Traditionally, ethylene oxide was been produced through a chlorohydrin process. This process was replaced by the complete oxidation process, pioneered by Theodore Lefort in 1931. Over the following years chemists have successfully synthesised other epoxides including propylene oxide. These Epoxides are incredibly versatile due to the addition of the oxygen atom on the tetrahedral ring, making the compound strained and unstable. Consequently the ring can be easily opened up, releasing abundant amounts of energy that can be used in further reactions, such as nucleophilic addition, hydrolysis and reduction. | + | Traditionally, ethylene oxide was been produced through a chlorohydrin process. This process was replaced by the complete oxidation process, pioneered by Theodore Lefort in 1931. Over the following years chemists have successfully synthesised other epoxides including propylene oxide. These Epoxides are incredibly versatile due to the addition of the oxygen atom on the tetrahedral ring, making the compound strained and unstable. Consequently the ring can be easily opened up, releasing abundant amounts of energy that can be used in further reactions, such as nucleophilic addition, hydrolysis, and reduction. |
Over time the production processes for ethylene oxide have evolved, incrementally improving efficiency and reducing waste.<sup>3</sup> This has demonstrated a chemical industry happy and willing to embrace change to improve their environmental impact, even if on occasion it is to the detriment of their bottom line. Thus, we believe that if we can develop an economically-feasible product we will be able to make a strong case for its replacement of contemporary processes. | Over time the production processes for ethylene oxide have evolved, incrementally improving efficiency and reducing waste.<sup>3</sup> This has demonstrated a chemical industry happy and willing to embrace change to improve their environmental impact, even if on occasion it is to the detriment of their bottom line. Thus, we believe that if we can develop an economically-feasible product we will be able to make a strong case for its replacement of contemporary processes. | ||
Revision as of 23:59, 17 September 2015
Overview & Motivation
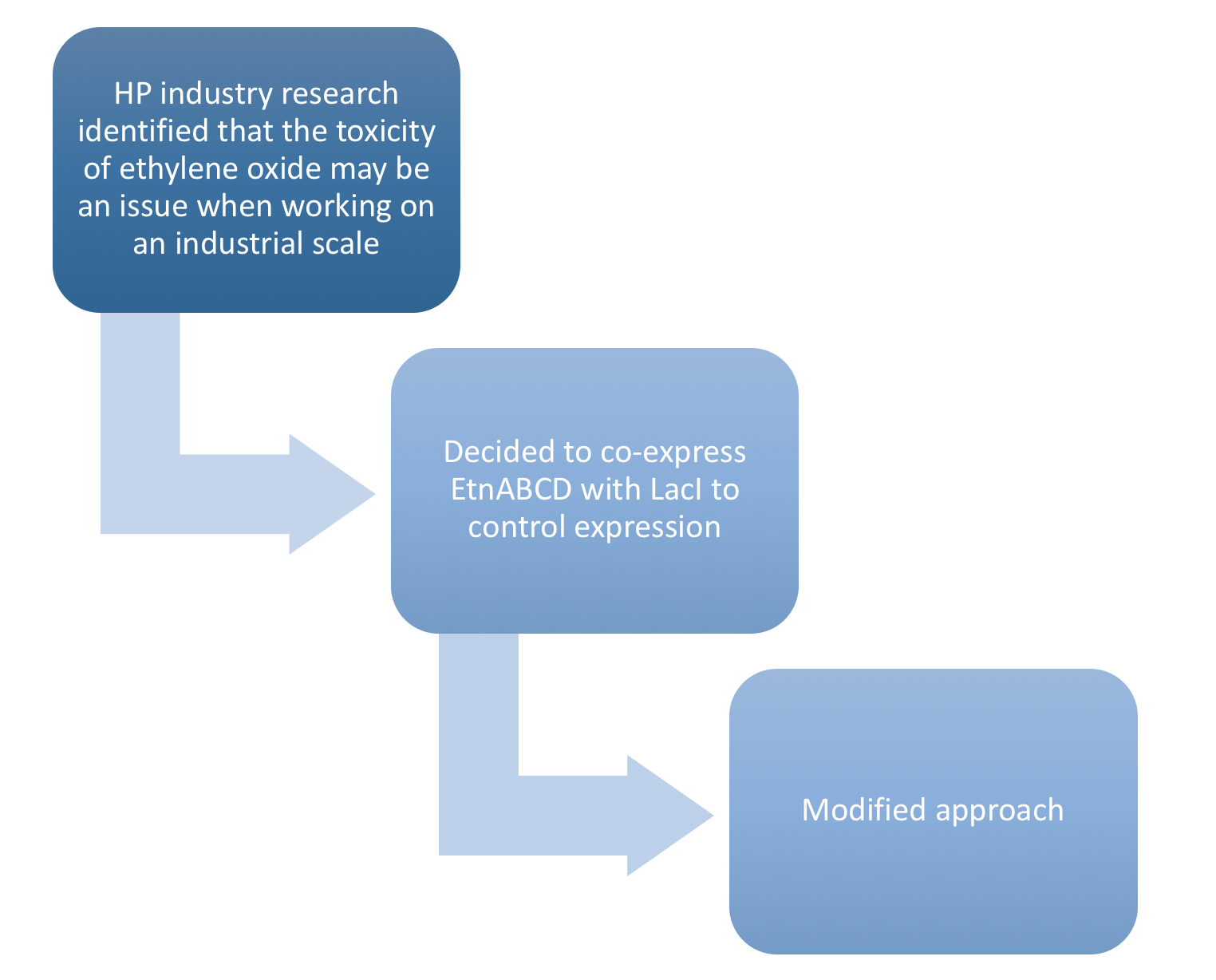
Our project has a very viable "real world application", and thus we created a Business Model and plan for the transition from laboratory experiment to a feasible product. We researched our target industry, assessed competitors, risks, and barriers to entry, and combined this information to create a comprehensive business report and pitch to any potential backers. Studying these problems from a business perspective provided influential insight into our iGEM project, and indeed the entire concept of our project is a direct result of the volatile, finite, and environmentally damaging methods of ethylene oxide production that are ubiquitous at present. In addition, through our research, we discovered that the amount of ethylene oxide a single cell had to produce in order to be a more efficient alternative than the current method of production would be toxic to the cell. Thus we needed to create a mechanism in which the expression could be cellularly monitored and repressed when the production of ethylene oxide nears the "danger zone". This lead directly to the inclusion of the LacI in our project, which became one of the submitted parts.
Summary
The purpose of this report is to provide an analysis of the development and commercial economic viability and likelihood of the MycoMimic product. This product was produced by the University of Sydney 2015 international Genetically Engineered Machine (iGEM) team, building on the work of Dr Nicholas Coleman at the University of Sydney.
Our project aims to produce ethylene oxide in a more efficient, environmentally friendly, and timely manner. Ethylene oxide is a prominent and versatile substance that is found in a plethora of products we all use daily. However, at present the production method of ethylene oxide is environmentally detrimental, expensive, and uses non-renewable resources as its reagents.1 A major focus of governments, industries, companies, and universities over the last few decades has been the creation of a more sustainable future. Indeed, in University of Sydney’s Environmental Sustainability Policy, there is an emphasis on encouraging and “integrating relevant environmental sustainability themes into courses and research” (15.B). Furthermore, numerous environmental and sustainability organisations, including the United Nations Environmental Program, have highlighted the need for greener and more sustainable alternative procedures for production of common chemical compounds.
We believe that the product developed in our project can significantly assist in decreasing carbon emissions and in creating a more sustainable future for all. The future demand for ethylene oxide is only going to increase as global population rises and countries such as China and India expand in wealth and industry. Consequently, in a time where the demand for ethylene oxide is increasing we simply cannot afford to continue using inefficient, environmentally damaging, and expensive methods of chemical synthesis.
Our Pitch
Bacterial production of ethylene oxide is a significantly better alternative to traditional chemical synthesis.
Current Situation
Ethylene oxide, one of the most abundantly produced chemical compounds, is derived from ethylene via an oxidation process. The sectors in which this compound is utilised include (but are not limited to) the pharmaceutical, medical, and manufacturing industries. The world production of ethylene oxide is \(17\times10^6\) metric tons2 and will only increase on the back of the expanding economies of countries such as China and India.
Due to the abundant use and versatility of ethylene oxide it is evident this compound is at present essential to our society: Thus, it is imperative we find a new avenue for production of this critical substance in an environmentally friendly and cost efficient manner.
Current Methods of Production
Traditionally, ethylene oxide was been produced through a chlorohydrin process. This process was replaced by the complete oxidation process, pioneered by Theodore Lefort in 1931. Over the following years chemists have successfully synthesised other epoxides including propylene oxide. These Epoxides are incredibly versatile due to the addition of the oxygen atom on the tetrahedral ring, making the compound strained and unstable. Consequently the ring can be easily opened up, releasing abundant amounts of energy that can be used in further reactions, such as nucleophilic addition, hydrolysis, and reduction.
Over time the production processes for ethylene oxide have evolved, incrementally improving efficiency and reducing waste.3 This has demonstrated a chemical industry happy and willing to embrace change to improve their environmental impact, even if on occasion it is to the detriment of their bottom line. Thus, we believe that if we can develop an economically-feasible product we will be able to make a strong case for its replacement of contemporary processes.
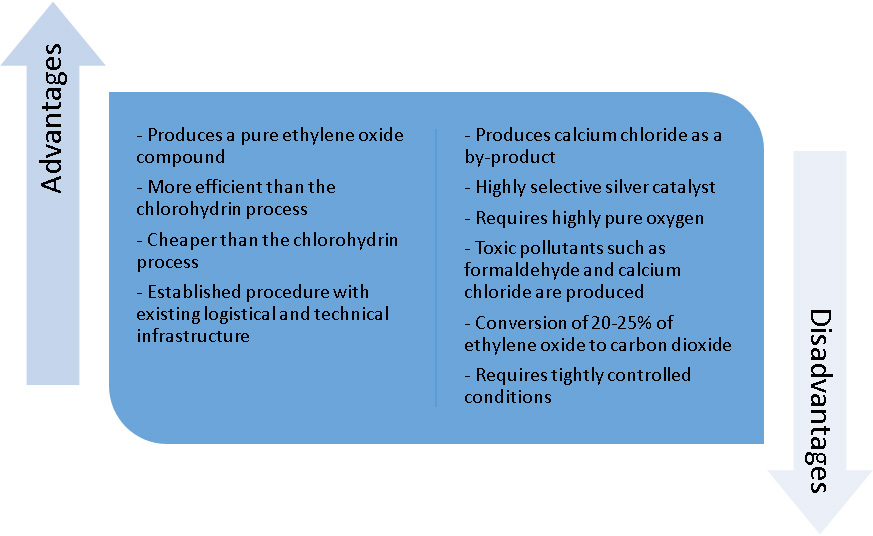
In Figure 2 the advantages and disadvantages of current methods of production are compared: Based on the numerous disadvantages of the complete oxidation process, which we believe far outweigh the advantages, it is evident a new, clean, green process needs to be created and implemented on an industrial scale.
Case Study: Production of Insulin by e. coli
Though MycoMimic hopes to break new ground in industrial synthesis, the application of bacteria for large-volume chemical synthesis of chemicals is not without precedent: One of the early breakthroughs in this field came in the development of an e. coli strain able to produce Insulin. This venture draws many parallels with our own, and thus serves as a case study against which we can compare5.
Prior to the application of bacterial synthesis Insulin was, much like ethylene oxide, produced by an inefficient and environmentally degrading method. This involved the processing of huge volumes of pig pancreases, with more than 8 tons necessary for the production of each kilogram of purified insulin.5 Due to this inefficiency researchers sought, as we do with MycoMimic, an alternative production pathway. This eventuated in the late 1970s, when after much research and development scientists finally demonstrated the ability to express a gene in e. coli which enabled it to produce human-viable insulin. This product, Humulin, represented the first recombinant DNA product the world had ever seen:5 An amazing breakthrough.
Production was rapidly scaled up to industrial levels, resulting in manufacturing plants containing thousands of 50,000 liter fermentation tanks. The processes these early pioneers developed inspired those used in our project: Antibiotic resistance is utilised to select out appropriate e. coli strains, which are then left to reproduce in an incubator over the course of some days. Once this reaches maximal capacity it is time to begin production, which is kick-started by the addition of an inducer to the broth. Without the means to break through the cellular membrane the Insulin accumulates inside the body of each bacterium, and thus maximal production is reached within the span of a few hours. Following this the bacteria are separated from their broth using a centrifuge, before being broken open to retrieve precursor compounds to Insulin. Though the bacteria's work is done, chemical adjustment and purification processes follow, eventually resulting in Insulin products perfect for human consumption.
Aside from the core industrial processes involved, many aspects of bacterial Insulin production may provide important lessons for our project. For example, in the Insulin process once fermentation tanks are populated (but before inducers are added), the bacterial population is sampled and checked for unexpected mutations:5 Discarding mutants at this stage not only minimises the possibility of undesireable end-products, but also reduces the time and material cost the overall process incurs due to these occasional mutations (which in biological systems are inevitable). Similar safety checkpoints will be necessary in any ethylene oxide synthesis process: Via their inclusion the risk of any physical threats (such as explosion of synthesised products) can be monitored, and financial and time costs due to errors in production may be minimised.
A Solution: MycoMimic
Monooxygenase enzymes are capable of performing this epoxide reaction, thus converting alkenes to epoxides safely and efficiently. These biological catalysts are renewable, non-toxic, biodegradable, and can be scaled up for large-volume production. Our product, MycoMimic is a genetically engineered Pseudomonas Putida bacterium which contains the enzyme ethene monooxygenase. With the insertion and successful expression of this enzyme, the bacterium can produce ethylene oxide.
Given MycoMimic is an easy product to work with, it can be used on a large and small scale. The only requirements for this product are the bacteria MycoMimic itself and a source of ethylene. Thus, both large and small industrial companies can use this product – creating a more accessible, transparent, and open market. We believe this is important as it creates competition in the market, which will lead to a more diverse and fair environment.
Based on lessons learned from prior implementations of bacterial synthesis procedures in industry, to bring MycoMimic into action as a substantive source of ethylene oxide we would require development of industrial plants on a large scale. Typically, synthesis is performed simultaneously in large collections of mid-sized fermentation tanks, though the mechanisms of this process can differ widely. For example, synthesis can involve bacteria producing and storing compounds in their interior, thus necessitating a batch's complete destruction during harvesting (see case studies). However, other methods instead implement secretion mechanisms, allowing a bacterium to deposit produced chemicals directly into the surrounding broth.5 This is as we would intend for MycoMimic's implementation, and thus some of the enabling technologies may already exist, particularly since we intend to achieve final expression in e. coli, which already finds extensive use in industry.
Risk analysis
Technical
It is imperative MycoMimic is closely monitored when it is scaled up to an industry level: Ethylene oxide is a highly flammable and reactive compounds. When working with it on a small scale in the laboratory, it poses minimal and controllable threats. However, when utilised at an industry and commercial scale it has the potential to be dangerous and harmful. Thus, only via close monitoring and regular security and safety checks can the producer ensure the potential for accidents is minimised. This also pushes the industrial technology required for MycoMimic away from that used in past biological synthesis processes, because with this new procedure will come new requirements for safety and reliability.
Our case study serves to illustrate another major technical challenge of MycoMimic's application on an industrial scale, which is that development of the necessary logistical and regulatory framework can provide a substantial challenge outside of the biological context. Complex process-engineering projects would thereby raise the overhead costs of implementation, and unforeseen challenges may provide time-consuming road blocks. Though the need for logistical development is mainstay in almost all industrial synthesis, as MycoMimic departs considerably from many existing synthesis procedures, this will likely provide a major challenge.
Financial
As with many projects, financial risks can provide obstacles that even the most creative scientific approach can struggle to overcome. In the business implementation process these risks can include failing to manage cost restraints and changes, and can quickly balloon when unforeseen complications arise. Though we would like to hope that industry players would be open to trialing MycoMimc as a means to reduce their environmental impact, in the real world this decision is likely to be made (for the most part) based on cost. Therefore, perhaps the greatest risk facing MycoMimic is a failure to become economically viable: If we cannot demonstrate a process which at least has the potential to be economically scaled up, it will be all but impossible for our new technology to be implemented on any meaningful scale. Similar obstacles have plagued innumerable past ventures, which can be seen in the plethora of biological solutions to important environmental problems that are unfortunately stalled by a lack of economic feasibility.
By continuing to promote the research and development of biological synthesis pathways we believe that even if MycoMimic does not achieve this economic feasibility, we will have taken important strides in laying the groundwork for similar technologies to be implemented in the future. With each such venture new research spotlights and studies the financial barriers in this field, and as we incrementally push these forwards we will see greater numbers of possibilities for the implementation of synthetic biology in industry emerge.
Another financial risk, which must be considered by businesses biological or otherwise, is an unstable market with increasing competition from other countries. In Australia, as one of the most economically prosperous nations in the Asia-Pacific region, great effort needs to be undertaken to ensure the financial aspect of the product is under control. For example, since employee wages and legal costs are in Australia larger than those in many competing regions, it is necessary that all care be taken in improving process efficiency and feasibility. Furthermore, competition can also arise in the form of new methods for production, such as that current used for crude oil. In the short term, competition from oil-companies may prove to be a risk. However, in the long-term, the financial advantages of MycoMimic are greater, as too are its prospects for minimisation of environmental impact. This is because companies are moving away from non-renewable resources, particularly in Australia where the investment in coal has dropped dramatically over the last 5 years.
Safety and Society
Though other processes have pioneered the implementation of genetically modified organisms to industrial synthesis, the community reaction in this regard may prove a risk. For example, it would not be unexpected that objections would be raised against the idea of engineered organisms producing highly explosive compounds. In this unlikely (but still possible) event, prevailing public opinion could result in unforeseen legal costs, slowed development of technologies, and even regulatory obstruction. It is therefore necessary to firstly establish the different views on this issue held throughout the community, and secondly to explain or market the product so that the consumers understand the benefits of MycoMimic, both to themselves and the environment.
This has been done to varying degrees of success by past synthetic biological ventures. For example, many producers of genetically modified food have systematically failed to inform the public of their benefits, along with their risks, which has developed widespread feelings of distrust. Though this has been overcome to some degree by stand-out success stories, such as that of Golden Rice, a number of misinformed campaigns continue which advocate cessation of most genetic modification practices. Thus, going forward it is essential that consumers are well informed (as we have aimed to do with our Human Practices project), such that they can make scientifically sound judgements upon these issues which play increasingly important parts in their lives.
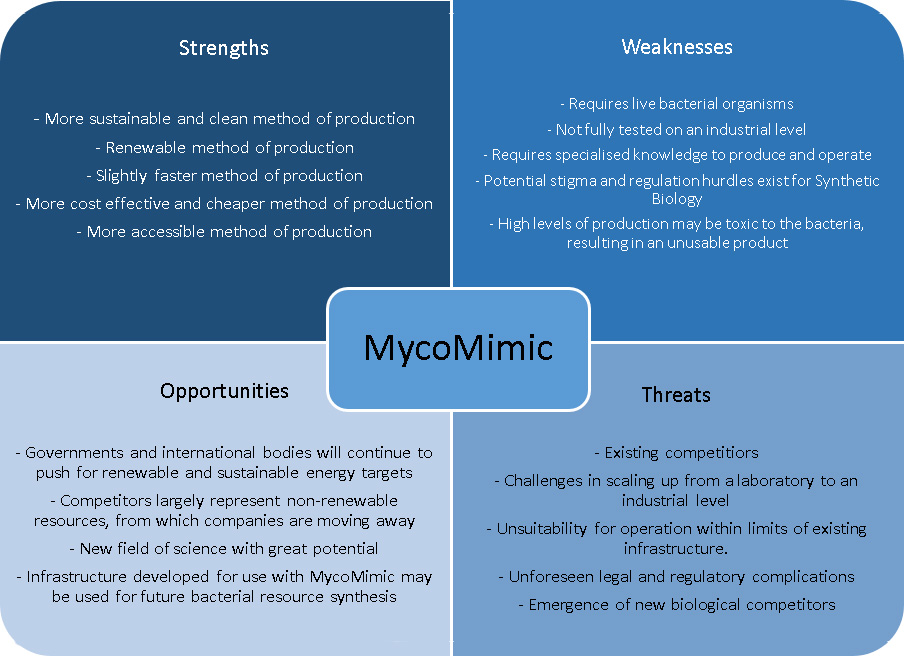
Product Analysis and Development
A SWOT analysis is used to assess the strengths, weaknesses, opportunities, and threats of this project. Building upon this analysis we can proactively plan an effective strategy that will focus on the strengths of this product while catering for weaknesses and possible threats.
A major advantage of MycoMimic is its capacity to be utilised on a large and small scale. Large companies can produce the thousands of tonnes of ethylene oxide necessary to supply an entire nation. Alternatively, smaller companies could produce ethylene oxide to supply only a state or a few communities. This is beneficial for a number of reasons: Firstly, it allows MycoMimic's utilisation in a flexible and changing market, a market which suits both large scale corporations and small organisations. This creates a safety net, allowing production to be varied in response to a changing unstable market. Secondly, the reduced barrier-to-entry and comparative ease of production provided by MycoMimic creates a more accessible market, preventing monopolies from forming in which the control of this important compound might fall into the hands of a few.
By considering our SWOT analysis (particularly the weaknesses identified), throughout project we made proactive changes to improve MycoMimic:
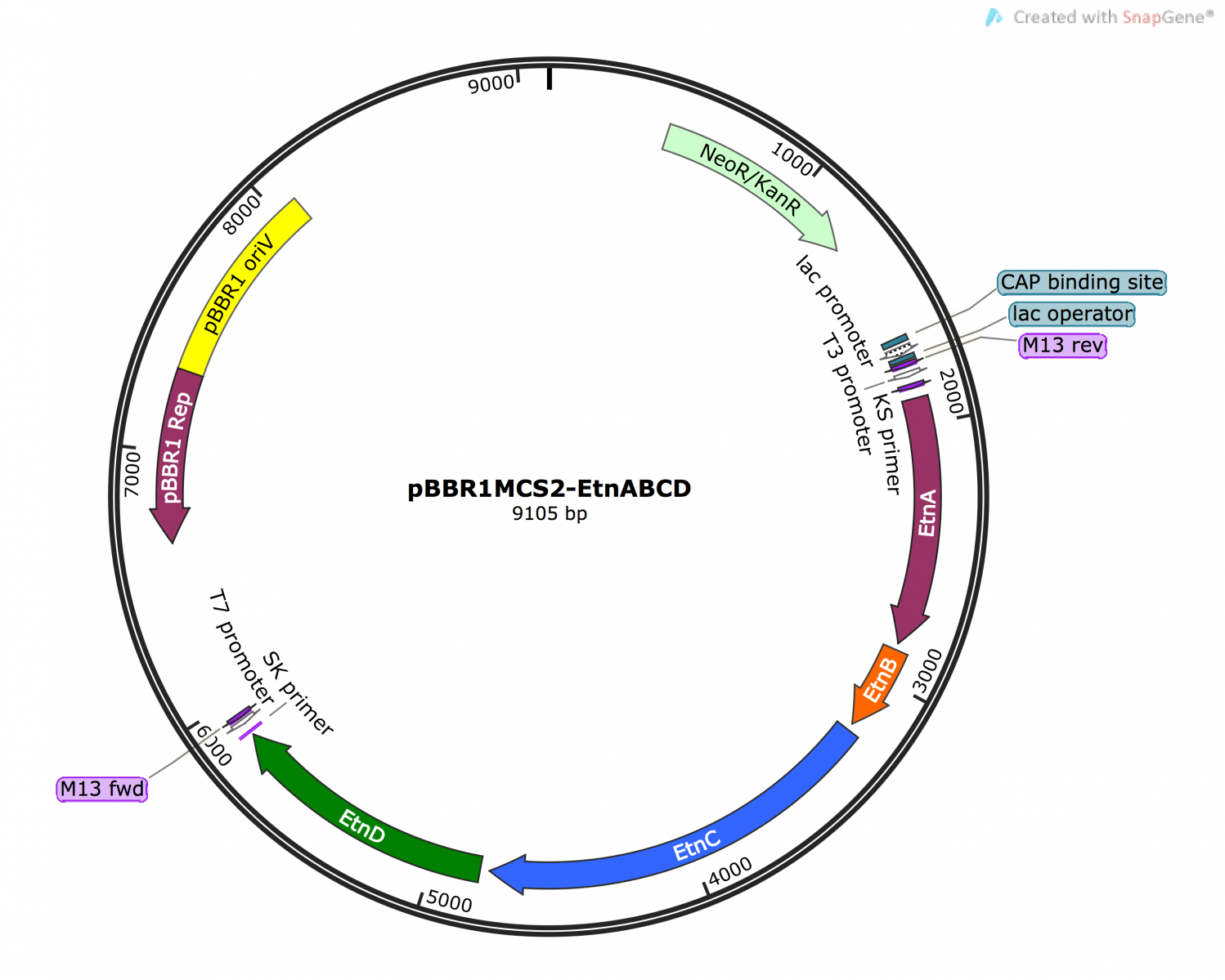
- To address the identified weakness of toxicity due to excessively high levels of protein production, we co-expressed the operon LacI which will stop the production of ethylene oxide before these levels are reached.
- To take advantage of the opportunity identified for governmental support, we engaged with the New South Wales state government, in return receiving funding for part of our project (see Attributions).
- To investigate the challenges and possibilities of scaling up production of MycoMimic, we discussed what would be involved, and what this might require, with a number of our sponsoring BioTechnology firms (see Attributions). By sharing their experiences of challenges faced in major BioTech projects they were able to help us develop an appreciation for threats and weaknesses that we might have otherwise not perceived. Encouraged by these discussions we set expression in e. coli as the eventual aim of our project, since this is the most common and effective host for production on industrial scales.
- As public concern and opposition plays a major part in all Synthetic Biological ventures, we were able to tailor our approach to Human Practices to address this. By sharing details of our business plans, particularly how they can lessen the environmental impact of industrial processes, we were able to garner considerable interest, and help to allay common (but often misinformed) fears held by the public.
- By identifying opportunities for expansion of our methodology to biological synthesis of other chemicals, our business plan played an important part in motivating our modelling research: Since many hurdles will need to be overcome with each synthesis pathway developed in the future, we designed adaptable tools for codon optimisation. These can help to open up new opportunities by facilitating the expression of a diversity of genetic circuits in industry-suitable bacteria.
Conclusion
As outlined, MycoMimic has the potential to be an incredibly effective, versatile, and necessary product in a time where the world is striving to be more environmentally sustainable and friendly. Many aspects of our project were motivated by consideration from a business perspective, which revealed the range of interlinked challenges faced in the implementation of synthetic biological technologies. Moving forwards after MycoMimic, much of our work has been completed with the necessity for future industry implementation in mind: By developing adaptive tools and methodologies, and by improving public understanding of these technologies, we hope that we have helped to enable a market more receptive to synthetic biological solutions to everyday problems.
Disclaimer – This proposal was written by the Sydney_Australia team as part of the International Genetically Engineered Machine competition. Its aim was to demonstrate the capacity and the MycoMimic project has to be applied to the real world and developed into a working product. However, it is not indicative of a final proposal that should be considered by businesses.
1 United States Environmental Protection Agency, 1986, Locating and estimating air emission from sources of ethylene oxide, viewed 20th of July 2015, http://www.epa.gov/ttnchie1/le/ethoxy.pdf (p. 11).
2 The Essential Chemical Industry, 2013, Epoxyethane (Ethylene Oxide), viewed 17th of August 2015, http://www.essentialchemicalindustry.org/chemicals/epoxyethane.html
3 Shell Global, 2015, Ethylene oxide/ethylene glycol (EO/EG) processes, viewed 20th of August 2015, http://www.shell.com/global/products-services/solutions-for-businesses/globalsolutions/refinery-chemical-licensing/petrochemical-technology/ethylene-oxide-processes.html
4 Sevas Education Society - Biotechnology, 2015, Manufacture of Ethylene Oxide, http://www.sbioinformatics.com/design_thesis/Ethylene_oxide/Ethylene-2520oxide_Methods-2520of-2520Production.pdf
5 Diabetes Forecast, 2015, Making Insulin: A behind-the-scenes look at producing a lifesaving medication, viewed 30th of August 2015, http://www.diabetesforecast.org/2013/jul/making-insulin.html