Difference between revisions of "Team:Goettingen/Experiments"
| Line 91: | Line 91: | ||
</td> | </td> | ||
<td valign="top" width="150"> | <td valign="top" width="150"> | ||
| − | <p>ad. | + | <p>ad. 1000 mL</p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 181: | Line 181: | ||
<td valign="top" width="97"> | <td valign="top" width="97"> | ||
<p> | <p> | ||
| − | + | 14 mL | |
</p> | </p> | ||
</td> | </td> | ||
| Line 193: | Line 193: | ||
<td valign="top" width="97"> | <td valign="top" width="97"> | ||
<p> | <p> | ||
| − | 7. | + | 7.5 mL |
</p> | </p> | ||
</td> | </td> | ||
| Line 217: | Line 217: | ||
<p>1g Yeast extract</p> | <p>1g Yeast extract</p> | ||
<p>3.5 ml 50% w/w Phytic acid</p> | <p>3.5 ml 50% w/w Phytic acid</p> | ||
| − | <p>0. | + | <p>0.2 g CaCl2</p> |
| − | <p>0. | + | <p>0.5 g MgSO4</p> |
<p>Adjust the pH to 7.2 with NaOH</p> | <p>Adjust the pH to 7.2 with NaOH</p> | ||
| − | <p>Ad | + | <p>Ad 2 L of Millipore H2O</p> |
| − | <p> | + | <p>32 g agar</p> |
<p>Autoclave</p> | <p>Autoclave</p> | ||
<p> </p> | <p> </p> | ||
| − | <p>After autoclaving the medium must cool down to ca. | + | <p>After autoclaving the medium must cool down to ca. 50˚C. Now glycerol, IPTG, BCIP and the respective antiobiotic can be added.</p> |
<p> </p> | <p> </p> | ||
<p>For 2 L medium:</p> | <p>For 2 L medium:</p> | ||
| − | <p> | + | <p>2 ml BCIP</p> |
| − | <p> | + | <p>2 ml 1M IPTG</p> |
| − | <p> | + | <p>2 ml Ampicllin or Kanamycin</p> |
| − | <p> | + | <p>40 mL Glycerol</p> |
<p>The media is now ready for plating</p> | <p>The media is now ready for plating</p> | ||
<p><br /> <strong>Result</strong>: On Sperber medium phosphatase-recombinant colonies should develop a distinct color blue after 2 days.</p> | <p><br /> <strong>Result</strong>: On Sperber medium phosphatase-recombinant colonies should develop a distinct color blue after 2 days.</p> | ||
| Line 240: | Line 240: | ||
<p style="text-align: center;"><span style="font-size: x-large; color: #888888;"><strong><span lang="EN-GB"></span></strong></span></p> | <p style="text-align: center;"><span style="font-size: x-large; color: #888888;"><strong><span lang="EN-GB"></span></strong></span></p> | ||
<p><span lang="EN-GB"> </span></p> | <p><span lang="EN-GB"> </span></p> | ||
| − | <p><span lang="EN-GB">To | + | <p><span lang="EN-GB">To 500 ml of LB Media add 7.5 g of Agar and 5 ml of Tributyrin and homogenize with a mixer.</span></p> |
<p><span lang="EN-GB">This culture medium must be directly sterilized by autoclaving at 121˚C for 20 min.</span></p> | <p><span lang="EN-GB">This culture medium must be directly sterilized by autoclaving at 121˚C for 20 min.</span></p> | ||
<p><span lang="EN-GB">If you wait too long it will be inhomogeneous again!</span></p> | <p><span lang="EN-GB">If you wait too long it will be inhomogeneous again!</span></p> | ||
| − | <p><span lang="EN-GB">When the medium cools down | + | <p><span lang="EN-GB">When the medium cools down to 50°C, the antibiotic can be added.</span></p> |
<p><span lang="EN-GB"> </span></p> | <p><span lang="EN-GB"> </span></p> | ||
<p><strong><span lang="EN-GB">Result:</span></strong><span lang="EN-GB"> Halo formation is visible around the positive clones.</span></p> | <p><strong><span lang="EN-GB">Result:</span></strong><span lang="EN-GB"> Halo formation is visible around the positive clones.</span></p> | ||
| Line 417: | Line 417: | ||
</td> | </td> | ||
<td valign="top" width="246"> | <td valign="top" width="246"> | ||
| − | <p>10µl</p> | + | <p>10 µl</p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 425: | Line 425: | ||
</td> | </td> | ||
<td valign="top" width="246"> | <td valign="top" width="246"> | ||
| − | <p> | + | <p>125 ng</p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 433: | Line 433: | ||
</td> | </td> | ||
<td valign="top" width="246"> | <td valign="top" width="246"> | ||
| − | <p> | + | <p>50 ng (1µl)</p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 441: | Line 441: | ||
</td> | </td> | ||
<td valign="top" width="246"> | <td valign="top" width="246"> | ||
| − | <p>1µl</p> | + | <p>1 µl</p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 449: | Line 449: | ||
</td> | </td> | ||
<td valign="top" width="246"> | <td valign="top" width="246"> | ||
| − | <p>xµl</p> | + | <p>x µl</p> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 457: | Line 457: | ||
</td> | </td> | ||
<td valign="top" width="246"> | <td valign="top" width="246"> | ||
| − | <p>20µl</p> | + | <p>20 µl</p> |
</td> | </td> | ||
</tr> | </tr> | ||
Revision as of 13:27, 18 September 2015
Media/Buffer
LB Medium
- Add the following components
|
NaCl |
10 g |
|
Yeast extract |
5 g |
|
Tryptone |
10 g |
|
H2O |
ad. 1000 mL |
- To obtain solid media add 15g/L agar.
- Autoclave at 121 oC for 20 min.
- The preferred antibiotic is added from a concentrated stock solution to an appropriate concentration (e.g Ampicilin is added to a final concentration of 100 µg/ml).
- To the liquid medium antibiotic is added upon usage.
- For the preparation of agar plates antibiotic is added after autoclaving the media and cooling it to 60 oC.
"Fat" LB Medium
Fat medium was used as culture medium for the induction of our cultures
|
Yeast extract |
40 g |
|
KH2PO4 (MW: 136.09) |
7.0 g |
|
Na2HPO4 x 12 H2O (MW: 358.14) |
15.14 g |
|
H2O dest. |
ad. 1000 mL |
pH 6.8 with NaOH (ca. 10mL 1M NaOH)
After autoclaving add:
|
50% Glycerine (sterile) |
14 mL |
|
24% MgSO4 x 7 H2O (sterile) |
7.5 mL |
Phosphatase Activity plates, Sperber media
A: Stock reagents
1M IPTG (4.76 g in 20ml Millipore-H2O) filter with blue 0.22 µm sterile filter and freeze at -20˚C
50mg/ml Kanamycin in Millipore H2O, filter with blue 0.22 µm sterile filter and freeze at -20˚C
100mg/ml Ampicillin in 50% ethanol, filter with blue 0.22 µm sterile filter and freeze at -20˚C
25mg/ml BCIP (in DMF) filter with blue 0.22 µm sterile filter and keep in a falcon tube wrapped with aluminum foil at 4˚C. BCIP: Biomol Nr. 2291 (MG 433.64)
Glycerol (99%), autoclaved and kept at room temperature
B: Sperber medium
1g Yeast extract
3.5 ml 50% w/w Phytic acid
0.2 g CaCl2
0.5 g MgSO4
Adjust the pH to 7.2 with NaOH
Ad 2 L of Millipore H2O
32 g agar
Autoclave
After autoclaving the medium must cool down to ca. 50˚C. Now glycerol, IPTG, BCIP and the respective antiobiotic can be added.
For 2 L medium:
2 ml BCIP
2 ml 1M IPTG
2 ml Ampicllin or Kanamycin
40 mL Glycerol
The media is now ready for plating
Result: On Sperber medium phosphatase-recombinant colonies should develop a distinct color blue after 2 days.
Esterase Activity plates, with 1% Tributyrin
To 500 ml of LB Media add 7.5 g of Agar and 5 ml of Tributyrin and homogenize with a mixer.
This culture medium must be directly sterilized by autoclaving at 121˚C for 20 min.
If you wait too long it will be inhomogeneous again!
When the medium cools down to 50°C, the antibiotic can be added.
Result: Halo formation is visible around the positive clones.
Cellulase activity plates
A: LB_ agar base
- Add the following components
|
Yeast extract |
5 g |
|
Tryptone |
10 g |
|
NaCl |
10 g |
|
H2O |
Add to 1 L |
|
Agar |
15 g |
- Make sure to put a stirrer in the bottle.
- Sterilize the culture medium by autoclaving at 121 oC for 20 min.
B: Substrate for cellulase screening
In 15 ml falcon tube, the substrate with final concentration 1% (w/v) is prepared in 96% (v/v) ethanol. For-example, to prepare 10 ml stock solution, weight 0.1 g of AZCL-HE-Cellulose and transfer it to 15 ml falcon tube, then add 96% (v/v) ethanol until the volume reached 10 ml.
The stock solution will be stored at +4 °C.
C: Prepare the agar plates
When the medium cools down to around 60 °C, put the bottle containing LB-agar base on a magnetic mixer. At the same time, disperse the substrate by flip the falcon tube several times. Then, pour the substrate into the LB-agar base gently until the substrate suspend evenly in the agar medium (For how much substrate should be poured, ask the supervisor). Other reagents like antibiotic can also be added. Ca. 3-5 ml substrate per Liter medium.
D: Preparation of agar plates contacting cellulose substrate
After dispersing substrate and adding antibiotic to LB-agar , pour the medium quickly with continuous stirring onto the plates and make sure to make a thin layer of medium. Let the plates to dry and store them at 4 °C.
E: Another method of pouring Substrate for cellulase screening on to the agar plates
Rather than using normal pouring method one can first prepare regular LB plates having just a thin layer of LB. After preparation of substrate for cellulase screening add the proper antibiotic to it and then pour this mixture on top of this thin LB layer with constant stirring. Let the plates to dry and then store at 4 °C.
1x TAE Buffer
1 x TAE Buffer
|
Tris |
24.22 g |
|
Acetic acid (100%) |
5.74 ml |
|
EDTA (0.5M, pH 8.0) |
10 ml |
|
H2O dest. |
ad. 5 L |
Cloning Methods
PCR product purification using QIAquick® PCR Purification Kit (QIAGEN)
- Add ethanol (96–100%) to Buffer PE before use.
- All centrifugation steps are carried out at 17900 g in a conventional table-top microcentrifuge at room temperature.
- Add 5 volumes Buffer PB to 1 volume of the PCR reaction and mix.
- Place a QIAquick column in a provided 2 ml collection tube.
- To bind DNA, apply the sample to the QIAquick column and centrifuge for 30–60 s. Discard flow-through and place the QIAquick column back in the same tube.
- To wash, add 0.75 ml Buffer PE to the QIAquick column, centrifuge for 30–60 s. Discard flow-through and place the QIAquick column back in the same tube.
- Centrifuge the QIAquick column once more in the provided 2 ml collection tube for 1 min to remove residual wash buffer.
- Place each QIAquick column in a clean 1.5 ml microcentrifuge tube.
- To elute DNA, add 50 μl water (40 – 60 oC) to the center of the QIAquick membrane, let the column stand for 1 min, and then centrifuge for 1 min.
PCR Gel extraction, peqGOLD Gel Extraction Kit
- Fractionate DNA fragments by running a agarose gel (Do not stain the gel with Ethidium bromide or expose the DNA to UV for too long).
- Add equal volume of Binding Buffer to the gel slice and incubate at 65ᵒC for 8 min. Vortex or mix every 2 to 3 min until the agarose dissolves completely. (0.2 g of gel equivalent to 0.2 ml)
- Pour the mixture into PerfectBind DNA column (which is placed in a 2 ml collection tube) and centrifuge for 1 min at 10000 x g.(max. 750 µl) Discard the flow-through and place the PerfectBind DNA column in the same tube. Repeat the steps if required.
- Add 300 µl of binding Buffer to the PerfectBind DNA column for the washing the contaminants and centrifuge for 1 min at 10000 x g. Discard the flow-through and place the column in the same tube
- Add 750 µl of CG Wash Buffer to the PerfectBind DNA column for the wash, incubate for 2 to 3 min and centrifuge for 1 min at 10000 x g. Discard the flow-through and place the column in the same tube. Repeat this step once more.
- Centrifuge the PerfectBind DNA column once more in the 2 ml collection tube for 1 min at 10000 x g to remove the residual wash buffer.
- Place the PerfectBind DNA column in a clean 1.5 ml eppendorf tube.
- Add 50 µl of pre-warmed sterile water to the PerfectBind DNA column and incubate for 2 to 3 min (normally in a 2 step process of 30 µl in first elution step and 20 µl in second elution step) and centrifuge at 5000 x g for 1 min.
- Store the purified DNA at -20ᵒC.
Blunt End Ligation in pJET1.2 vector –Clone JET PCR Cloning Kit– (Thermo Scientific)
- Set up the following componentson ice for the ligation reaction.
|
Components |
Volume |
|
2x Reaction buffer |
10 µl |
|
Blunt end PCR product |
125 ng |
|
pJET 1.2 Plasmids |
50 ng (1µl) |
|
T4 DNA Ligase |
1 µl |
|
Water(RNase free) |
x µl |
|
Total Volume |
20 µl |
- Vortex briefly and centrifuge for 3-5 sec at 5000 rpm.
- Carry out the ligation at 20°C for 20 min at room temperature (22-25°C) and then place in ice for 5 min and use for transformation immediately or store at -20°C.
Cloning principle:
- pJET1.2 is a linearized cloning vector which accepts inserts from 6 bp to 10 kb. Since the 5’-ends of the vector contain phosporyl groups, therefore phosphorylation of the PCR products is not required.
- Optimal insert/vector ratio is 3:1. (0.15 pmol ends of insert and 0.05 pmol ends of vector)
- Optimal PCR product quantity for ligation reaction is to be calculated from the Kit protocol, For the length of 2500 bp of PCR product, to have 0.15 pmol ends of the PCR product in the ligation reaction 125 ng of the PCR product should be used.
- For PCR products more than 3 kb, ligation can be prolonged to 30 min.
Sticky End T4 Ligation (Thermo Scientific)
T4 Ligation - Thermo Fisher Scientific - sticky end ligation
1. Prepare the following reaction mixture:
|
Linear vector DNA |
20 ng |
|
Insert DNA |
80 ng |
|
10x T4 DNA Ligase buffer |
2 µl |
|
Thermo Scientific T4 DNA Ligase (Cat #EL0016) |
1 U |
|
Nuclease free H2O |
Add to 20 µl |
2. Incubate 1 hour at RT
3. Heat inactivation of T4 DNA Ligase at 65 °C for 10 minutes or at 70 °C for 5 min
TOPO® Cloning protocol usingChampion™ pET Directional TOPO® Expression Kits (Thermo Fisher Scientific)
- You will perform TOPO® Cloning in a reaction buffer containing salt. Note that the amount of salt added to the TOPO® Cloning reaction varies depending on whether you plan to transform chemically competent cells or electrocompetent cells.
- Set up the TOPO® Cloning reaction depending on the transformation method.
|
Reagents |
Chemically Competent E. coli |
Electrocompetent E. coli |
|
Fresh PCR product |
0.5 to 4 μl (15 – 40 ng) |
0.5 to 4 μl (15 – 40 ng) |
|
Salt Solution |
1 μl |
--- |
|
Dilute Salt Solution (1:4) |
--- |
1 μl |
|
Sterile Water |
add to a final volume of 5 μl |
add to a final volume of 5 μl |
|
TOPO® vector 1 |
1 μl (15 – 20 ng) |
1 μl (15 – 20 ng) |
|
Total Volume |
6 μl |
6 μl |
- Mix reaction gently and incubate for 15 - 30 minutes at room temperature (22-23°C).
- Place the reaction on ice and proceed with the transformation or store at - 20 °C.
- Before transformation into electrocompetent E.coli the salt is removed via microdialysis. For more information refer to transformation in electrocompetent BL21 E.coli.
Plasmid transformation into chemically competent E. coli
- Thaw chemically competent E.coli cells (BL21 or TOP10) on ice.
- Add 100 – 250 ng of ligation product. Mix gently, do not pipette up and down.
(add 30 – 60 ng pET 101 construct depending on the cloning protocol).
- Place on ice for 5 – 15 min.
- Heat shock 45-50 seconds at 42 °C.
- Incubate 2-5 min on ice.
- Add 300 - 500 μl of LB medium.
- Incubate at 37°C for 1 hour with shaking.
- Plate 100 – 150 μl on prewarmed LB plate containing the proper antibiotic. Centrifuge the rest for 2 min at 5000 xg. Decant the supernatant and move the tube against hard surface to suspend the pellets in the residual medium. Plate the cells on another LB plate containing the proper antibiotic.
- Incubate LB plates at 37°C overnight.
- Check plates for colonies growth.
- For positive control set up another transformation using an equal amount of a circular plasmid possessing the proper antibiotic resistance.
Electroporation of BL21 cells with pJET_RFP
- Apply microdialysis for 30 -45 min by applying the transformation mixture (plasmid+buffer) on (Millipore® MF-Millipore™ DNA Fillter Paper for Dialysis of DNA and Proteins – capitol scientific) after placing the membrane on sterile water.
- Defreeze 40 µl aliquots of chemically competent cells on ice
- Mix 50-200 ng DNA with the cells. (desalted)
- Transfer the culture without any air bubbles into pre-cooled electroporation cuvettes (40 µl maximum) and incubate on ice for 10 minutes.
- Electroporate using the electroporator with 1.25 mV, 5 decharge time.
- Immediately after electroporation, transfer 300 µl room temperature LB medium on top of the cells and transfer it into an 1.5ml fresh E-cup
- Incubate the culture for 1 hour at 37°C and 150 rpm
- Spread a 100 µl from the dilution series (10-3 to 10-6) on a pre-warmed LB plate containing ampicillin
- Incubate the plates overnight at 37°C
Plasmid Extraction - using QIAprep Spin Miniprep Kit (QIAGEN)
This protocol describes the purification of plasmid DNA from 5 ml overnight cultures of E. coli grown in LB medium using the QIAprep Spin Miniprep Kit (QIAGEN)
- Add the provided RNase A solution to buffer P1, mix, and store at 4 oC.
- Add ethanol (96–100%) to Buffer PE before use.
- All centrifugation steps are carried out at 17900 x g (13000 rpm) in a conventional table-top microcentrifuge at room temperature.
- Add the proper antibiotic with the proper concentration to 5 ml LB medium (e.g Ampicilin is added to a final concentration of 100 µg/ml).
- Inoculate the medium with the desired E.coli strain and incubate overnight at 37 oC with agitation (150 rpm).
- Pellet the overnight culture by centrifugation at 8000 rpm (6800xg) for 3 min at room temperature.
- Resuspend pelleted bacterial cells in 250 μl Buffer P1 and transfer it to a microcentrifuge tube.
- Add 250 μl Buffer P2 and mix thoroughly by inverting the tube 4–6 times. Do not allow the lysis reaction to proceed for more than 5 min.
- Add 350 μl Buffer N3 and mix immediately and thoroughly by inverting the tube 4–6 times.
- Centrifuge for 10 min at 17900 x g in a table-top microcentrifuge.
- Apply the supernatant to the QIAprep spin column by decanting. Centrifuge 60 s. Discard the flow-through.
- Wash the QIAprep spin column by adding 500 μl Buffer PB and centrifuging for 60 s. Discard the flow-through.
- Wash QIAprep spin column by adding 750 μl Buffer PE and centrifuging for 30–60 s.
- Discard the flow-through, and centrifuge for an additional 1 min to remove residual wash buffer.
- Place each QIAquick column in a clean 1.5 ml microcentrifuge tube.
- To elute DNA, add 50 μl water (40 – 60 oC) to the center of the QIAquick membrane, let the column stand for 1 min, and then centrifuge for 1 min.
Plasmid Extraction - using peqGOLD Plasmid Miniprep Kit I (PEQLAB Technologies)
Select few TOP10/BL21 E.coli transformed colonies from the LB Amp plates containing the propagated transformed colonies and inoculate them into liquid LB medium (5 ml) containing preferred antibiotic of desired concentration in each tube.
Incubate the LB tubes with the transformed colonies at 37°C in a shaker for about 12 – 15 hours (overnight) with agitation (150 rpm).
Extract the plasmids from the incubated LB cultures using the peqGOLD Plasmid Miniprep Kit I.
Centrifuge the culture at 10000 x g for 2 min to obtain the pellet and repeat the process until the culture is completely centrifuged. Store the pellet of 1 ml of the culture at -20°C for future use.
Resuspend the pellet in 250 µl of Solution I of the Kit (which is normally kept at 4°C because of the RNase) and vortex until the pellet is resuspended.
Add 250 µl of Solution II to the resuspended mixture and gently mix by inverting and rotating the tubes 6 -10 times to obtain a cleared lysate. Incubate the mixture for 2 min to obtain optimum results.
Add 350 µl of Solution III to the cleared lysate and gently mix by inverting the tubes 6 -10 times until a flocculent white precipitate is formed. Centrifuge at 10000 x g for 10 min at room temperature.
Transfer the clear supernatant to a fresh PerfectBind DNA Column in a 2 ml Collection Tube. Centrifuge the Column with the Collection Tube for 1 min at 10000 x g at room temperature. Discard the flow-through liquid.
Add 500 µl of PW Plasmid buffer to the PerfectBind DNA Column in the Collection Tube and centrifuge for 1 min at 10000 x g. Discard the flow-through.
Add 750 µl of DNA Wash buffer to the PerfectBind DNA Column in the Collection tube and centrifuge for 1 min at 10000 x g. Discard the flow-through. Repeat this step to obtain optimum results.
Place the PerfectBind DNA Column in the Collection tube and centrifuge for 2 min at 10000 x g to dry the column matrix. This step is essential to remove ethanol from the column.
Place the PerfectBind DNA Column into a fresh 1.5 ml Eppendorf tube. Add 50 µl of pre-warmed sterile deionized water directly to the binding matrix in the PerfectBind DNA Column and centrifuge for 1 min at 5000 x g to elute the DNA.
Discard the PerfectBind DNA Column and store the eluted plasmid DNA at -20°C.
Check the concentration of the plasmids by using a NanoDrop and note down the values for future experiments.
Competent Cells
Preparation of competent E.coli cells
- Method 1: Low amount of competent cells
Materials:
Buffer RF1 (150 ml): 1.44 g RbCl
1.16 g MnCl2.4H2O
3.6 ml Potassium acetate (1M)
pH 7.5 with acetic acid
Buffer RF2 (80 ml): 1.6 ml MOPS (0.5 M) stock solution
PH 6.8 with NaOH
0.096 g RbCl
0.88 g CaCl2.2H2O
Procedure
- Inoculate a 4 ml culture either with a single colony or with a cryoculture of the desired E.coli strainand incubate the culture with agitation overnight at 37°C
- Inoculate a 300 ml shake flask containing 100 ml LB medium with the overnight culture to an OD600 of 0.05 and grow the culture at 37°C until the OD600is about 0.3
- Transfer the cells into two 50 ml falcon tubes, incubate the cultures for 15 minues on ice and harvest the cells by centrifugation for 15 min at 5000 rpm and 4°C. Discard the supernatants.
- Resuspend the cells in 1/3 of the original volume (~16 ml/50 ml) of buffer RF1, incubate the cells again on ice and harvest the cells by centrifugation for 15 min at 5000 rpm and 4°C. Discard the supernatants.
- Resuspend the cells in 4 ml of buffer RF2 and incubate the suspensions for 15 min on ice. Prepare the Eppendorf tubes and liquid nitrogen.
- Put 0.4 ml of the cell suspension into the Eppendorf reaction tubes and freeze the cells by transferring them immediately to the liquid nitrogen. Store the competent cells at -80°C
- Method 2 (High amount of competent cells, time consuming)
Materials
TB 3.46 g Piperazine-N,N'-bis(2-ethanesulfonic Acid) Pipes, 11 mM
2.2 g CaCl2.2H2O (15 mM)
18.64 g KCl (250 mM)
PH 6.7, autoclave, add 55 ml MnCl2 (1M, sterile) to a final volume of 1 l
SOB-Mg 20 g/l tryptone (2%)
5 g/l Yeast extract (0.5%)
0.58 g/l NaCl (10 mM)
0.186 g/l KCl (2.5 mM)
SOB SOB-Mg
10 mM MgCl2
10 mM MgSO4
LB liquid medium
DMSO (Dimethyl sulfoxide)
Procedure:
- Inoculate a 20 ml culture either with a single colony or with the cryoculture of the desired E.coli strainand incubate the culture with agitation for 20 h at 28°C
- Inoculate a 250 ml SOB medium supplemented in a 2 l shake flask and grow the cells to an OD600 of 0.5- 0.9 (20-20 h) at 18°C and 200-250 rpm
- Incubate the whole flask for 10 min on ice. Collect the cells by centrifugation for 10 min at 4°C and 5000 rpm. Resuspend the cells in 80 ml of ice-cold TB and incubate them for 10 min on ice. Collect the cells by centrifugation for 5 min at 5000 rpm.
- Resuspend the cells in a 20 ml of ice-cold TB. Add DMSO to a final concentration of 7% (1.4 ml) and gently shake the falcon tube.
- Transfer 0.2 ml aliquots into labelled eppendorf reaction tubes and freeze the cells in liquid nitrogen. Store the cells at –80°C.
Transformation Efficiency Kit, RFP construct (iGEM)
Before using our competent E. coli TOP10 cells in the important experiments, we used the Transformation Efficiency Kit to test the efficiency of our competent cells!
The kit includes five vials of each different DNA concentration: 50pg/μl, 20pg/μl, 10pg/μl, 5pg/μl, 0.5pg/μl of purified DNA from BBa_J04450 (RFP construct) in plasmid backbone pSB1C3.
Protocol as distributed by iGEM (modified)
Spin down the DNA tubes from the Transformation Efficiency Kit to collect all of the DNA into the bottom of each tube prior to use. A quick spin of 20-30 seconds at 8000-10000 rpm will be sufficient. Note: There should be 50 µL of DNA in each tube sent in the Kit.
Thaw competent cells on ice. Label one 2.0 ml microcentrifuge tube for each concentration and then pre-chill by placing the tubes on ice.
Pipet 1 µL of DNA into each microcentrifuge tube. For each concentration, use a separate tube.
Pipet 50 µL of competent cells into each tube. Flick the tube gently with your finger to mix. Incubate on ice for 30 minutes. Pre-heat heating block now to 42°C.
Heat-shock the cells by placing onto the heating block for 1 minute.
Immediately transfer the tubes back to ice, and incubate on ice for 5 minutes. This helps the cells recover.
Add 200 µL of LB media per tube, and incubate at 37°C for 1 hour. Prepare the LB-Cam (Chloramphenicol) agar plates during this time: label them.
Pipet 20 µL from each tube onto the appropriate plate, and spread the mixture evenly across the plate. Do triplicates (3 each) of each tube if possible, so you can calculate an average colony yield. Incubate at 37°C overnight. Position the plates so the agar side is facing up, and the lid is facing down.
Count the number of colonies on a light field or a dark background, such as a lab bench. Use the following equation to calculate your competent cell efficiency. If you've done triplicates of each sample, use the average cell colony count in the calculation.
(colonies on plate) / ng of DNA plated x 1000ng/µg
Note: The measurement "ng of DNA plated" refers to how much DNA was plated onto each agar plate, not the total amount of DNA used per transformation. You can calculate this number using the following equation:
1 µL x concentration of DNA (refer to vial) x (volume plated / total reaction volume)
Results
Competent cells should have an efficiency of 1.5x108 to 6x108 cfu/µg DNA, where "cfu" means "colony-forming unit" and is a measurement of cells.
Protein Extraction and Purification
Protein Extraction (French Press) and Purification (Protino® Ni-IDA 2000 His-Tag protein purification, Macherey-Nagel)
1 x LEW (Lysis-Equilibrium-Wash) Buffer:
|
NaH2PO4 x 2 H2O (MW: 156.07 g/mol) |
7.8g |
|
NaCl (MW: 58.44 g/mol) |
17.5g |
|
H20 dest. |
ad. 1000mL |
Adjust to pH 8.0 using NaOH.
1 x Elution Buffer:
|
NaH2PO4 x 2 H2O (MW: 156.07 g/mol) |
7.8g |
|
NaCl (MW: 58.44 g/mol) |
17.5g |
|
Imidazole (MW: 68.08 g/mol) |
17.0g |
|
H20 dest. |
ad. 1000mL |
Adjust to pH 8.0 using NaOH.
1.) Cell extraction by French Press:
- Resuspend pellet in 20mL 1x LEW Buffer from the kit
- Take a small sample (10µl) for microscopy
- Press 4 times with a French Press at High Pressure and collect the flow through
- Take a small sample (10µl) and analyse both before and after press samples under the microscope. Look for inclusion bodies.
- Centrifuge pressed samples at 4°C and 13000 rpm for 45 minutes, keep supernatant
2.) Protino® Ni-IDA 2000 His-Tag protein purification (Macherey-Nagel)
- Wet Ni-IDA column with 4mL of 1x LEW buffer and discard flow through
- Run supernatant from the cell extraction through the column and collect flow through (cell extract, store at 4°C)
- Wash column 3 times with 4ml 1x LEW Buffer
- Collect each flow through (wash 1-3) and store at 4°C
- Elute protein 3 times with 3mL Elution Buffer (contains Imidazole)
- Collect each elution and store fractions at 4°C
- Quantify protein content by Bradford measurement (see Bradford assay)
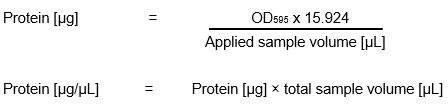
Bradford Assay
Bradford Assay
For calculating protein concentrations of an unknown sample the traditional way is to use a standard curve that is generated from known protein standards. Readily available protein standards contain bovine serum albumin (BSA) or bovine gamma globulin (γ-globulin, IgG). The calculated result is an estimation of protein concentration since Bradford protein assays do show significant protein-to-protein variation.
For our Bradford assay we got an already prepared standard solution with a factor of 15.924.
Caution: Store the Bradford solution in the dark, it is light sensitive.
Sample preparation:
Measurement takes place against 20 μL water (blank).
Blank: 20 μL + 1 mL Bradford solution
Samples: 1-20 μL (depending on protein concentration, max. 2 mg/mL) + 19-0 μL water + 1 mL Bradford solution
- Prepare photometer cuvettes for technical triplicates of all samples + one blank
- Preheat photometer light and set it to 595 nm
- Pipet the samples (plus water) and the blank into the cuvettes
- Pipet 1 mL Bradford solution on top, carefully homogenize by pipetting up and down
- Avoid air bubbles
- Incubate the cuvettes in the dark for 5 minutes
- Immediately measure the samples at 595 nm
Calculation of protein concentration:
Activity Screens
Esterase activity test
Materials:
Substrate- 1mM 4-nitrophenyl butyrate prepared in Na-Phosphate buffer (pH8.0)
Protein sample
Procedure:
- Add 2 µl of the protein sample to 2ml of the substrate and incubate for 5 minutes
- The colour change from white/transparent to yellow is observed which indicates the presence of the enzyme
Phosphatase activity test
Materials:
- Freshly prepared AAM Solution (Acetone, 5N H2SO4, 10 mM Ammonium molybdate, 2:1:1 v/v)
- 50 mM Sodium acetate buffer, pH 5.0
- 100 mM Pyrophosphate
- 1 M Citric acid
- Protein sample
Procedure:
- Set a heating block to 37°C or 40°C
- Place 2 mL reaction tubes for each sample (technical triplicates) plus one blank in the heating block
- Pipet 250 µL of 50mM Sodium acetate buffer in all reaction tubes
- Add 40 µL of protein sample
- Add 10 µL of 100 mM Pyrophosphate to the tubes
- Mix the tubes by inverting
- Incubate on the heating block for 30 minutes
- Add 1.5 mL of AAM solution and homogenize by inverting the tubes
- Add 100 µL 1 M Citric acid
- If phosphatase is present, the colour will change from white/transparent to yellow
- Measure the colour strength in a photometer at 355 nm
CAUTION: Make sure that the buffer you used for protein extraction does not contain any phosphate components. Otherwise the test will be false positive!
Cellulase activity screening
- Streak Competent Top10 E. coli cells or Competent BL21 cells transformed with appropriate vector containing cellulase construct onto agar plates containing cellulose substrate.
- Incubate plates at 37oC for minimum 3-4 days (timing can be extended depending on bacterial strain).
- After 3-4 days look for plates having clear zone of activity around cellulose substrate.
Restriction Controls
Aan I (Psi I ) - thermo fisher scientific - restriction control protocol
- Add the following components
|
Purified Plasmid |
1 µg |
|
10X Buffer Tango |
1 µl |
|
Ana I (Psi I) (10 U/L) |
0.5 µl |
|
Nuclease free H2O |
Add to 10 µl |
- Incubate at 37 oC for 2 hours
- For inactivation incubation at 65°C for 20 min.
Double digestion restriction control
- Add the following components
- For 10 µl reaction add 1 µl of 10x Tango buffer, for 20 µl reaction add 2 µl of 10x Tango buffer and vice versa.
- Incubate at 37 oC for 2 hours.
- For inactivation incubation at 80°C for 5min.
- Check the products on 0.8% agarose gel.
Double digestion restriction control
Sac I and Pvu II - thermo fisher scientific - restriction control protocol
|
Purified Plasmid |
800 ng |
|
Sac I (2x) |
2µl |
|
Pvu II (1x) |
1µl |
|
10x Tango buffer |
2 µl |
|
Nuclease free H2O |
Add to 20 µl |
Restriction control using fast and slow digestion enzymes
Restriction control using fast digestion enzymes
Sac I and HindIII - thermo fisher scientific - restriction control protocol
- Add the following components
|
Purified Plasmid |
500-1000 ng |
|
Sac I |
1 µl |
|
Hind III |
1 µl |
|
Nuclease free H2O |
Add to 10 µl |
- Incubate at 37 oC for 30 min.
- For inactivation incubation at 65°C for 5 min.
PST I - thermo fisher scientific - restriction control protocol
- Add the following components
|
Purified Plasmid |
500-1000 ng |
|
FD buffer |
1 µl |
|
PST I |
1 µl |
|
Nuclease free H2O |
Add to 10 µl |
- Incubate at 37 oC for 30 min.
- Products were checked on 0.8% agarose gel.
Restriction control using slow digestion enzymes
Bsm I - fermentas - restriction control protocol
- Add the following components
|
Purified Plasmid |
500 ng |
|
Red buffer |
1 µl |
|
Bsm I |
1 µl |
|
Nuclease free H2O |
Add to 8 µl |
- Incubate at 37 oC for 3 hours.
- Products were checked on 0.8% agarose gel.
Scafoldin Restriction control
Scaffoldin Restriction Control using the restriction enzyme BsmI
Set up the reaction as follows,
|
Components |
Quantity |
|
Nuclease free Water |
15 µl |
|
Red Buffer 10X |
2 µl |
|
DNA |
1000 ng (2 µl) |
|
BsmI |
1µl |
|
|
20 µl |
Incubate the mixture for 2 hours at 37ᵒC.
Perform the heat inactivation step for the reaction mixture for 10 min at 65ᵒC.
Esterase Restriction Control
Esterase Restriction Control
|
Nuclease Free Water |
15µl |
|
R Buffer 10X |
2µl |
|
DNA (up to 1µg) |
2µl |
|
XhoI (10U/µl) |
1µl |
|
PstI (10U/µl) |
1µl |
Incubate 20min at 37˚C and inactivate 5min at 80˚C.
Phosphatase Restriction Control
Phosphatase Restriction Control
|
Nuclease Free Water |
14µl |
|
Fast digestion Buffer 10X |
2µl |
|
DNA ( up to 1µg) |
2µl |
|
SacI (10U/µl) |
1µl |
|
KpnI(10U/µl) |
1µl |
Incubate 2 hours at 37˚C and inactivate 20min at 80˚C.
PCR Preparation Methods
Colony PCR
Colony PCR
- Pick a colony with a tip and re suspend it in 5ul LB
- Prepare the PCR mix as follows:
|
Water |
12ul |
|
Taq Buffer |
2ul |
|
MgCl |
2ul |
|
dNTPs |
0.5 µl |
|
Primer Forward |
1ul |
|
Primer Reverse |
1ul |
|
Taq Polymerase |
1.5 µl |
- Program:
Initial denaturalization: 98˚C, 5 min
Denaturalization: 96˚C, 45 s
Annealing: 70˚C, 30 s
Elongation: 72˚C, 25 s
Phusion PCR
This protocol describes PCR procedure using Phusion High-Fidelity DNA Polymerase. (Thermo Fisher Scientific).
• The annealing rules are different from many common DNA polymerases (such as Taq DNA polymerases).
•Use 15–30 s/kb for extension. Do not exceed 1 min/kb.
•Phusion DNA Polymerases produce blunt end DNA products.
•All components and reaction mix are placed on ice.
- add the following components, mix and spin down.
|
Component |
20 µL rxn |
50 µL rxn |
Final conc. |
|
H2O |
add to 20 µL |
add to 50 µL |
|
|
5X Phusion HF Buffer |
4 µL |
10 µL |
1X |
|
10 mM dNTPs |
0.4 µL |
1 µL |
200 µM each |
|
Forward primer |
X µL |
X µL |
0.5 µM |
|
Reverse primer |
X µL |
X µL |
0.5 µM |
|
Template DNA |
15 -20 ng |
30 - 50 ng |
|
|
(DMSO, optional)* |
(0.6 µL) |
(1.5 µL) |
3% |
|
Phusion DNA Polymerase |
0.2 µL |
0.5 µL |
0.02 U/µL |
* Addition of DMSO is recommended for GC-rich amplicons. DMSO is not recommended for amplicons with very low GC % or amplicons that are > 20 kb.
- adjust the thermocycle to the following isntructions and start the PCR.
|
Cycle step |
Tempreature oC |
Time |
Cycles |
|
Initial Denaturation |
98 |
30 s |
1 |
|
Denaturation |
98 |
5–10 s |
30 - 35 |
|
Annealing |
* |
10–30 s |
|
|
Extension |
72 |
15–30 s/kb |
|
|
Final extension |
72 |
5-10 min |
1 |
* The optimal annealing temperature for Phusion DNA Polymerase may differ significantly from that of Taq-based polymerases. Always use the Tm calculator and instructions on our website (www.thermoscientific.com/pcrwebtools) to determine the Tm values of primers and optimal annealing temperature. The Phusion DNA Polymerase has the ability to stabilize primer-template hybridization. As a basic rule, for primers > 20 nt, anneal for 10–30 seconds at a Tm +3°C of the lower Tm primer. For primers ≤ 20 nt, use an annealing temperature equal to the Tm of the lower Tm primer.
- for Templates > 2.5 kb and primers with low stability apply the following program
|
Cycle step |
Tempreature oC |
Time |
Cycles |
|
Initial Denaturation |
98 |
30 s |
1 |
|
Denaturation |
98 |
10s |
30 - 35 |
|
Annealing |
* |
15s |
|
|
Extension |
72 |
1 min 30 s |
|
|
Final extension |
72 |
10.5 min |
1 |
- check the PCR product on 0.8% agarose gel and store at - 20 celsius degrees.
Sequencing
Protocol for Sanger sequencing
- Sequencing reaction setup
|
Template |
DNA amount |
|
PCR products |
10 ng/100 bp (if larger 1.5 kb 150 ng) |
|
Plasmids |
300 ng |
|
Forward primer (5pmol/ µl ) |
1 µl |
|
Reverse primer (5pmol/ µl ) |
1 µl |
|
Nuclease free H2O |
ad. 5 µl |
|
Total volume |
5 µl |
- Forward and reverse primers should be added in 2 individual sample tubes!
Overnight Sanger Sequencing
- Forward and reverse primers should be added in 2 individual sample tubes!
Sanger sequencing reaction setup for overnight
|
Components |
Volume |
|
Purified Plasmid |
800 ng |
|
Forward primer (1:10) |
3 µl |
|
Reverse primer (1:10) |
3 µl |
|
Nuclease free H2O |
ad. 15 µl |
|
Total volume |
15 µl |
Fluorescence Microscopy
RFP microscopy
- Prepare 0.8 % agarose with water and boil it up.
- Pipet 500 μL onto a slide and press another one on top. Let the agarose cure between both slides.
- Carefully remove the upper slide and put the sample name in one corner the one with the agarose.
- Dilute the desired cell cultures 1:10 up to 1:50 (depends on thickness) and remember to prepare also a positive (if available) and negative control.
- Pipet 10-20 μL of the diluted culture on the agarose and put the cover slip on top.
- Perform fluorescence microscopy with the right filter.
- If successful, take pictures of the red fluorescing E. coli.
- Note: Agarose has a slight self-fluorescence.
RFP microscopy
To check if the transformed E.coli TOP10 show red fluorescence, the culture was examined by fluorescence microscopy. The RFP DsRed filter was used (excitation at 536 nm, emission at 582 nm). To prevent the cells from floating around they are fixed in 0.8 %.
Preparation of slides