Difference between revisions of "Team:Austin UTexas/Project/Problem"
| Line 1: | Line 1: | ||
{{Austin_UTexas}} | {{Austin_UTexas}} | ||
| − | + | = Problem: Genetic Instability = | |
| − | + | == Background == | |
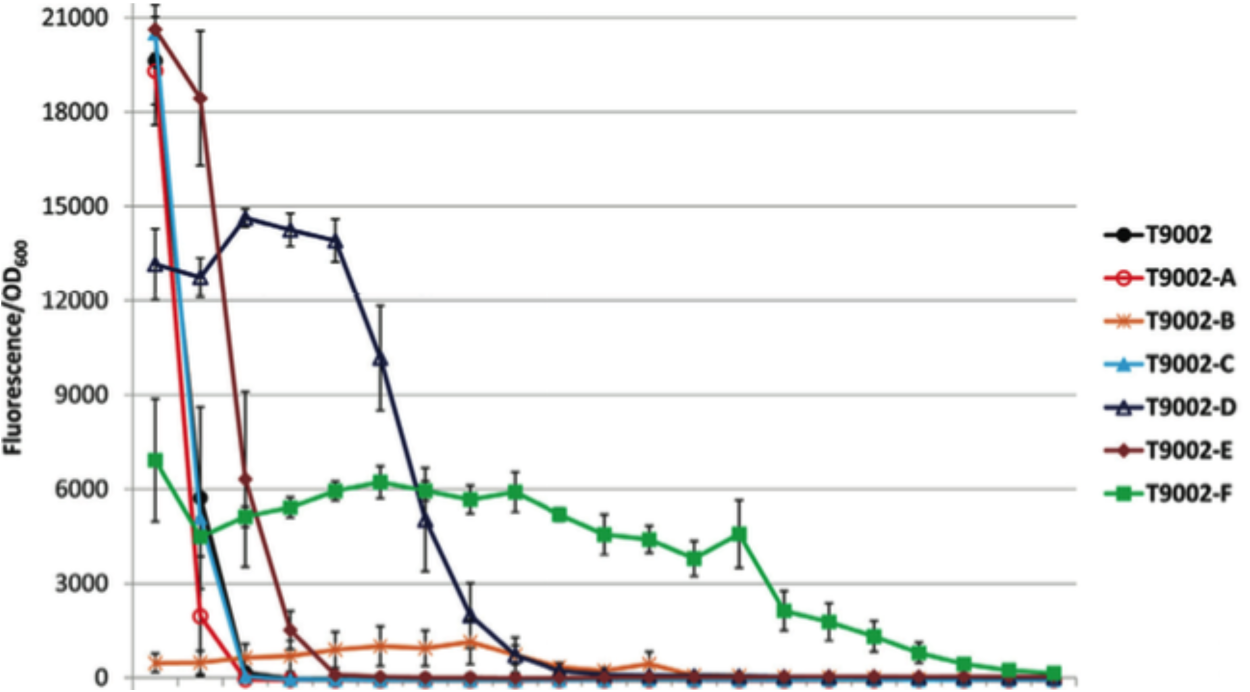
[[Image:2015_Austin_UTexas_sleightpaperfig.png|300px|thumb|right|Evolutionary stability of T9002 (black) and T9002 (color) re-engineered circuits with AHL - Image credit to Sean C. Sleight <i>et al.</i>]] | [[Image:2015_Austin_UTexas_sleightpaperfig.png|300px|thumb|right|Evolutionary stability of T9002 (black) and T9002 (color) re-engineered circuits with AHL - Image credit to Sean C. Sleight <i>et al.</i>]] | ||
| − | One of the major | + | One of the major issues facing genetic engineering is the longevity of genetic devices once inserted into organisms. An organism can be modified, but that does not ensure that the modification will last.<b> Subsequent generations of modified organisms often lose or “break” the genetic device through mutations.</b> Breaking a plasmid or an inserted device often decreases the metabolic load of that organism giving it a competitive advantage by enabling it to allocate cell resources toward replication. This allows them to dominate a population over some time. |
Previous research has determined several factors that contribute to the breaking of genetic devices. One major factor is the relative fitness of organisms without the device over those with it. Strongly expressed devices will break faster than less strongly expressed devices, since they have a greater metabolic load on the organism. Similarly, genes that code for 'costly' proteins that may interfere with other cellular functions in ways that are toxic are more likely to break. | Previous research has determined several factors that contribute to the breaking of genetic devices. One major factor is the relative fitness of organisms without the device over those with it. Strongly expressed devices will break faster than less strongly expressed devices, since they have a greater metabolic load on the organism. Similarly, genes that code for 'costly' proteins that may interfere with other cellular functions in ways that are toxic are more likely to break. | ||
| − | Another major factor that contributes to device stability is the rate of mutations leading to device failure. If a device includes DNA sequences that act as mutational hotspots it may be very unstable. | + | Another major factor that contributes to device stability is the rate of mutations leading to device failure. If a device includes DNA sequences that act as mutational hotspots, it may be very unstable. The [[http://barricklab.org/django/efm Evolutionary Failure Mode calculator]] developed by the Barrick lab identifies DNA sequences that are prone to breaking, such as simple sequence repeats and long stretches of a single nucleotide. The EFM calculator gives sequences a relative instability prediction (RIP) score, and higher RIP scores indicate more sequences where the gene is likely to break. |
=== Project and Motivation === | === Project and Motivation === | ||
| − | Our | + | Our projects focused on monitoring device stability and determining what kinds of sequences and sequence features contribute to device instability. <b>We wish to identify and better characterize sequences that contribute to the relative instability of genetic devices so they can be used to predict device longevity, as well as be taken into account when creating and modifying such devices</b>. In the spring of 2015, UT iGEM team members helped by the UT Austin Freshman Research Initiative stream "Hijacking Cell Factories for Synthetic Biology" utilized the EFM calculator to identify sequences within fluorescent protein coding genes that were more likely to break, or mutate. We used this information to form hypotheses and determine which fluorescent protein coding genes in our inventories are the most and least evolutionary stable. |
Over the summer, three plasmids were constructed: YFP + Medium RBS; Medium Promoter (BBa_K608006), Super-folder YFP + BBa_K608006, and Enhanced YFP + BBa_K608006. These three plasmids were used to transform four strains of bacteria (Top-10, MDS-42, BL-21 (DE3), BW-25113). We hoped that growing these transformed strains in culture and monitoring their fluorescence and stability would better elucidate the patterns in the sequences that contribute to the instability of genetic devices. Understanding this could lead to the active modification of devices to remove these 'risky' elements, and thus increase the longevity of genetically modified organisms. | Over the summer, three plasmids were constructed: YFP + Medium RBS; Medium Promoter (BBa_K608006), Super-folder YFP + BBa_K608006, and Enhanced YFP + BBa_K608006. These three plasmids were used to transform four strains of bacteria (Top-10, MDS-42, BL-21 (DE3), BW-25113). We hoped that growing these transformed strains in culture and monitoring their fluorescence and stability would better elucidate the patterns in the sequences that contribute to the instability of genetic devices. Understanding this could lead to the active modification of devices to remove these 'risky' elements, and thus increase the longevity of genetically modified organisms. | ||
{{Austin_UTexas_Footer}} | {{Austin_UTexas_Footer}} | ||
Revision as of 20:47, 18 September 2015
Problem: Genetic Instability
Background
One of the major issues facing genetic engineering is the longevity of genetic devices once inserted into organisms. An organism can be modified, but that does not ensure that the modification will last. Subsequent generations of modified organisms often lose or “break” the genetic device through mutations. Breaking a plasmid or an inserted device often decreases the metabolic load of that organism giving it a competitive advantage by enabling it to allocate cell resources toward replication. This allows them to dominate a population over some time.
Previous research has determined several factors that contribute to the breaking of genetic devices. One major factor is the relative fitness of organisms without the device over those with it. Strongly expressed devices will break faster than less strongly expressed devices, since they have a greater metabolic load on the organism. Similarly, genes that code for 'costly' proteins that may interfere with other cellular functions in ways that are toxic are more likely to break.
Another major factor that contributes to device stability is the rate of mutations leading to device failure. If a device includes DNA sequences that act as mutational hotspots, it may be very unstable. The http://barricklab.org/django/efm Evolutionary Failure Mode calculator developed by the Barrick lab identifies DNA sequences that are prone to breaking, such as simple sequence repeats and long stretches of a single nucleotide. The EFM calculator gives sequences a relative instability prediction (RIP) score, and higher RIP scores indicate more sequences where the gene is likely to break.
Project and Motivation
Our projects focused on monitoring device stability and determining what kinds of sequences and sequence features contribute to device instability. We wish to identify and better characterize sequences that contribute to the relative instability of genetic devices so they can be used to predict device longevity, as well as be taken into account when creating and modifying such devices. In the spring of 2015, UT iGEM team members helped by the UT Austin Freshman Research Initiative stream "Hijacking Cell Factories for Synthetic Biology" utilized the EFM calculator to identify sequences within fluorescent protein coding genes that were more likely to break, or mutate. We used this information to form hypotheses and determine which fluorescent protein coding genes in our inventories are the most and least evolutionary stable.
Over the summer, three plasmids were constructed: YFP + Medium RBS; Medium Promoter (BBa_K608006), Super-folder YFP + BBa_K608006, and Enhanced YFP + BBa_K608006. These three plasmids were used to transform four strains of bacteria (Top-10, MDS-42, BL-21 (DE3), BW-25113). We hoped that growing these transformed strains in culture and monitoring their fluorescence and stability would better elucidate the patterns in the sequences that contribute to the instability of genetic devices. Understanding this could lead to the active modification of devices to remove these 'risky' elements, and thus increase the longevity of genetically modified organisms.