Team:Sydney Australia/Results
Results Overview
Aim
We aimed to express the enzyme ethene monoxygenase enzyme in Pseudominas Putida and show the ethylene to ethylene oxide oxidation reaction. This was done by:
- Codon Harmonisation (using our inhouse algorithm TransOpt) of Mycobacterium chubuense ethene MO (EtnABCD) to match Pseudomonas putida's translation profiles.
- Expression of EtnABCD in P. putida
- Co-expression of E.coli GroEL-GroES to enhance folding to ensure highest possible activity
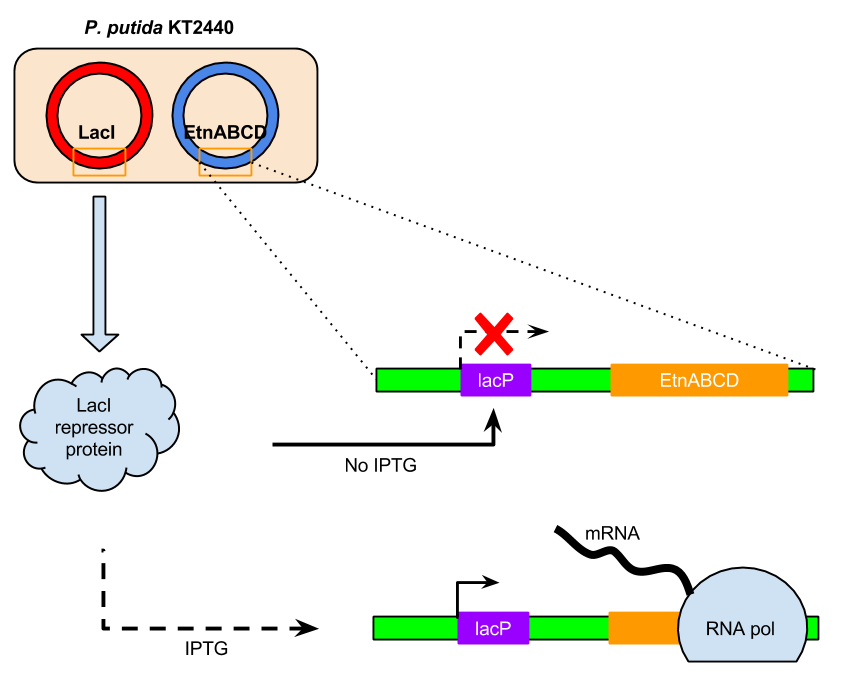
- Co-expression of LacIQ15 (LacI with constitutive promoter Q15) to control EtnABCD expression in P.putida
- Experimental validation of TransOpt via fluorescence assays using Bacillus subtilis fluorescent protein (BsFP)
Experimental design
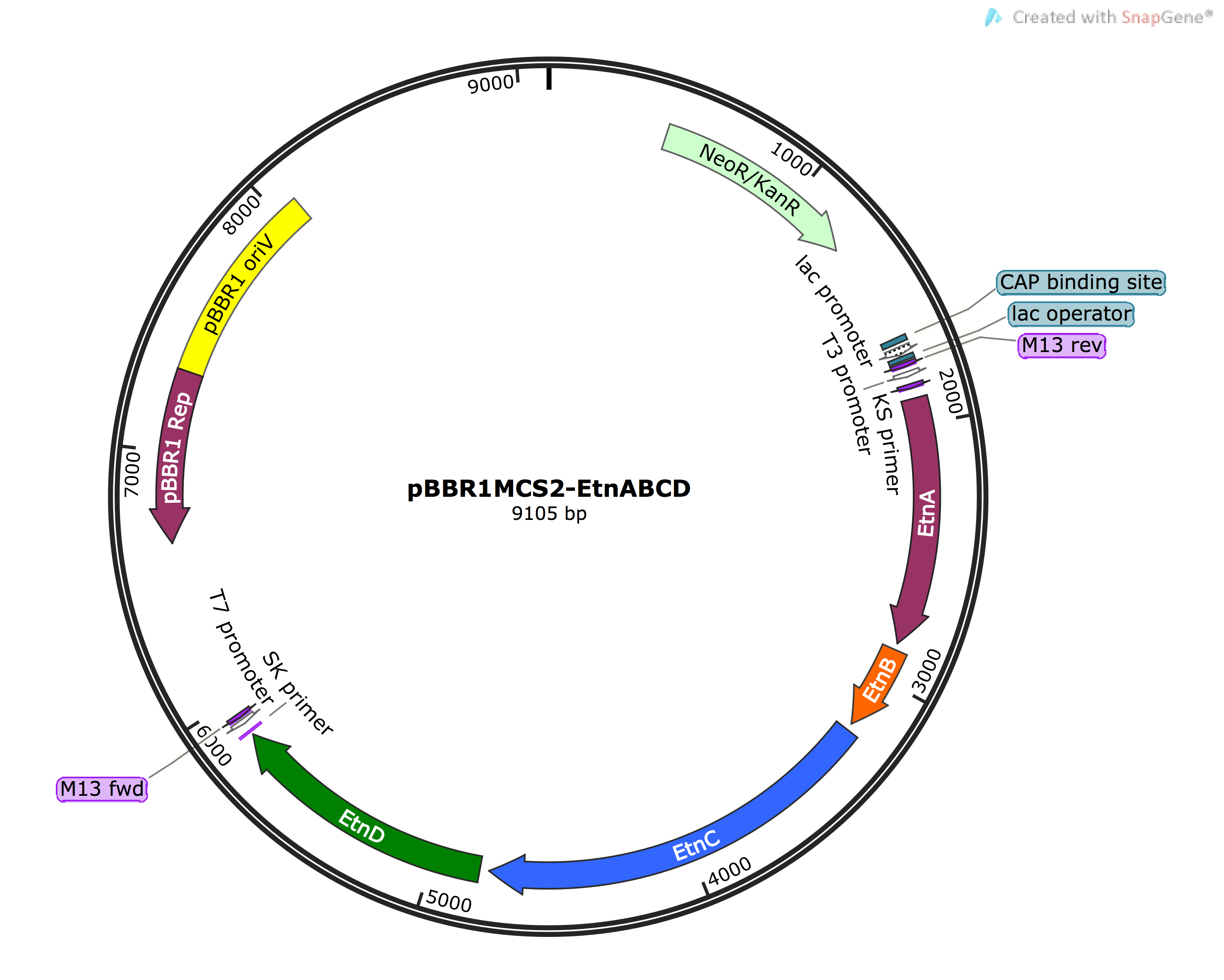
- Cloning EtnABCD Gblocks into pBBRMCS2 vector via Golden Gate Assembly and digestion/ligation
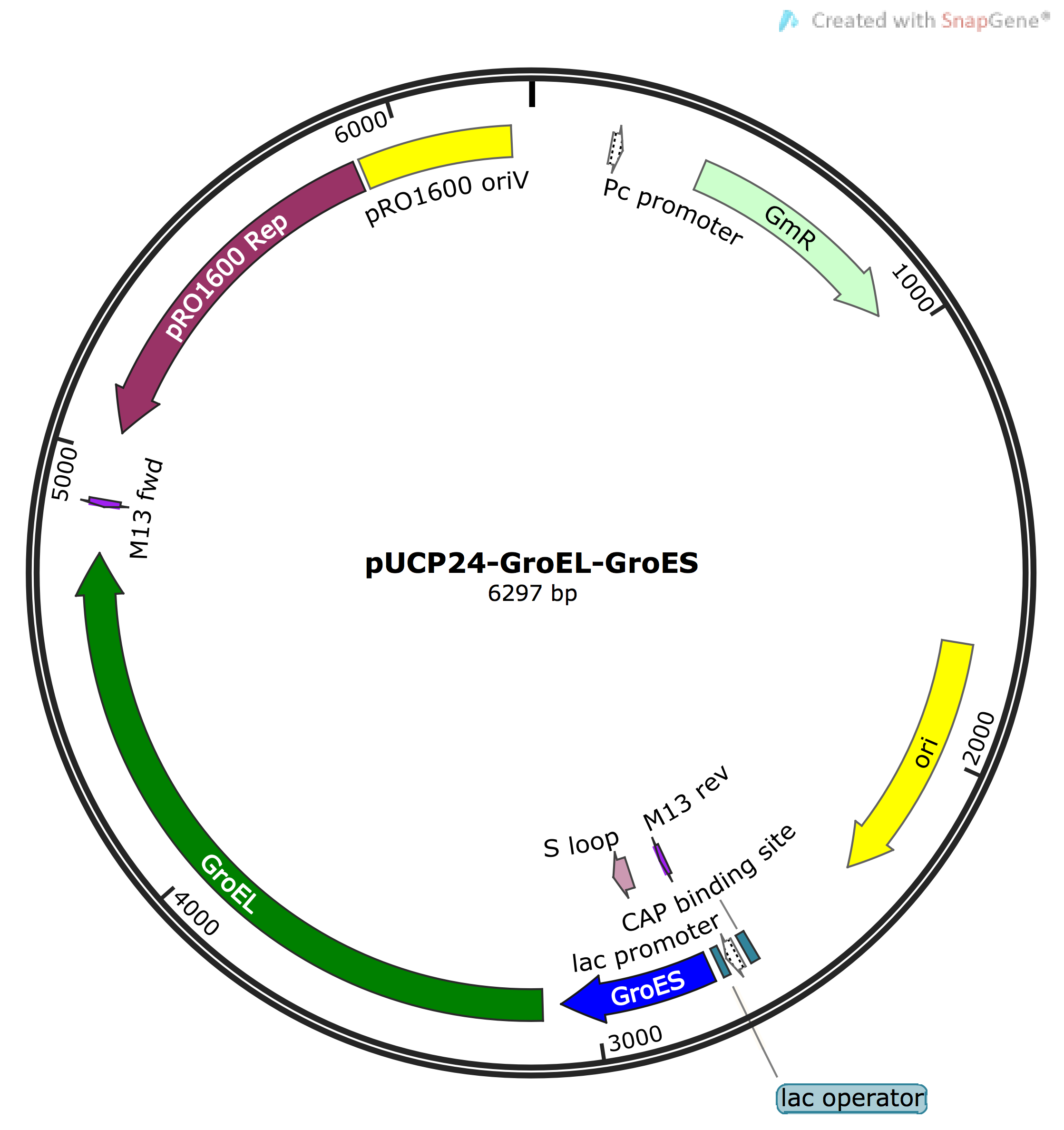
- Cloning GroEL-GroES into pUCP24 vector via PCR amplification from E.coli genome and digestion/ligation
- Cloning LacIQ15 into pSB1C3 (note that it carries its own promoter via PCR amplification and digestion/ligation)
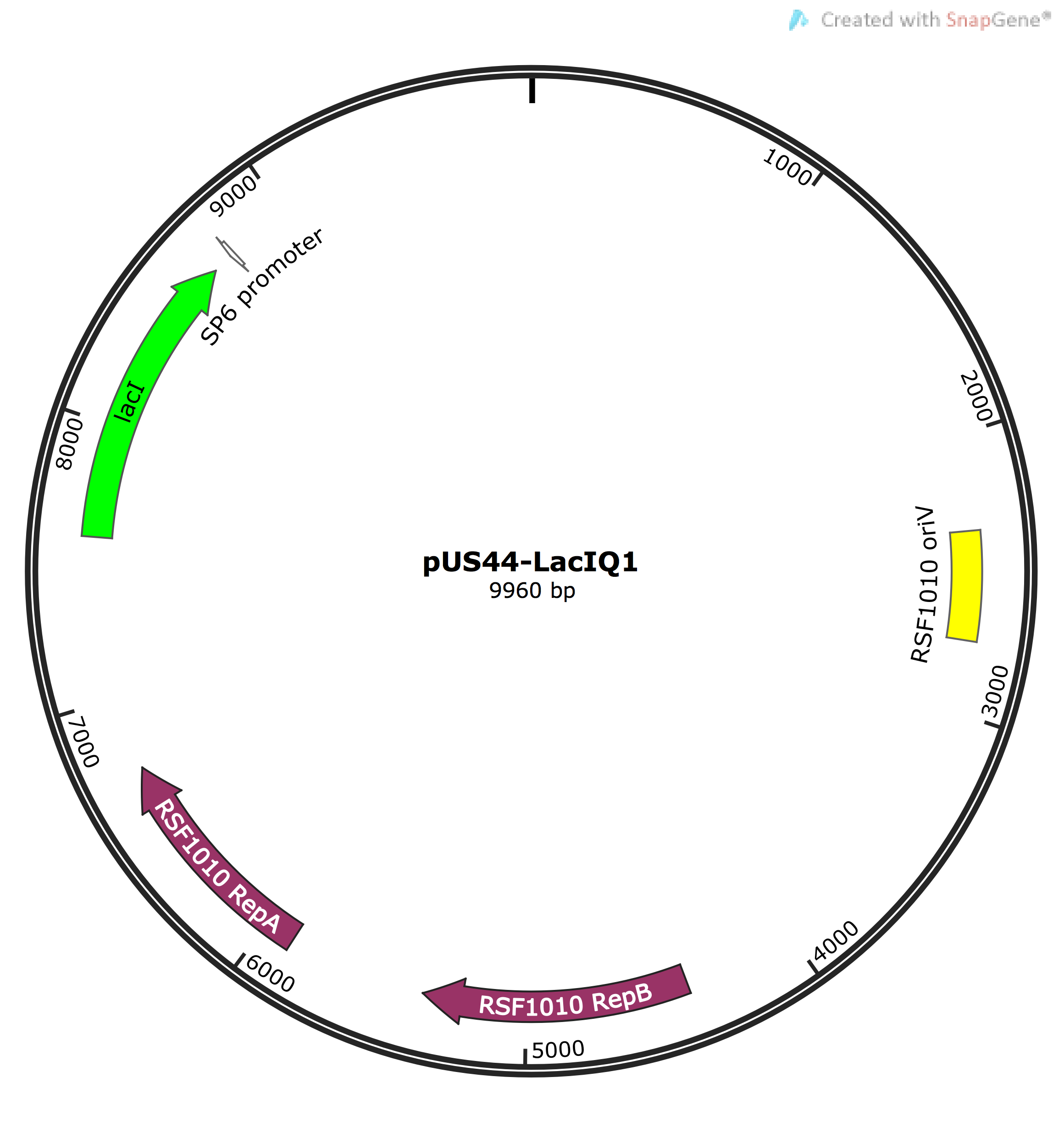
- Cloning LacIQ15 into pUS44 vector for co-expression with EtnABCD (note that pUS44 has different selectable marker so makes screening for presence of both EtnABCD and LacI easier)
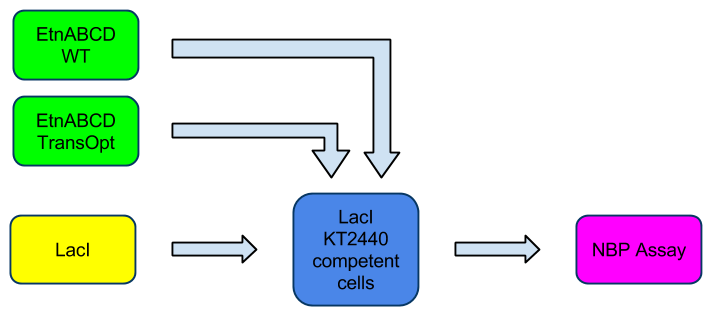
- Inducing EtnABCD expression in P. putida
- Assessing if EtnABCD was functional and producing ethylene oxide via a Nito-benzyl-pyridine (NBP) assay to detect the presence of ethene oxide
Summarised results
- NBP assay was not successful in detecting ethene oxide after induction of P.putida bearing LacI and EtnABCD, which shows that the enzyme does not work as expected
- LacIQ15 inhibited LacZ activity (lac operon in general) in the absence of IPTG
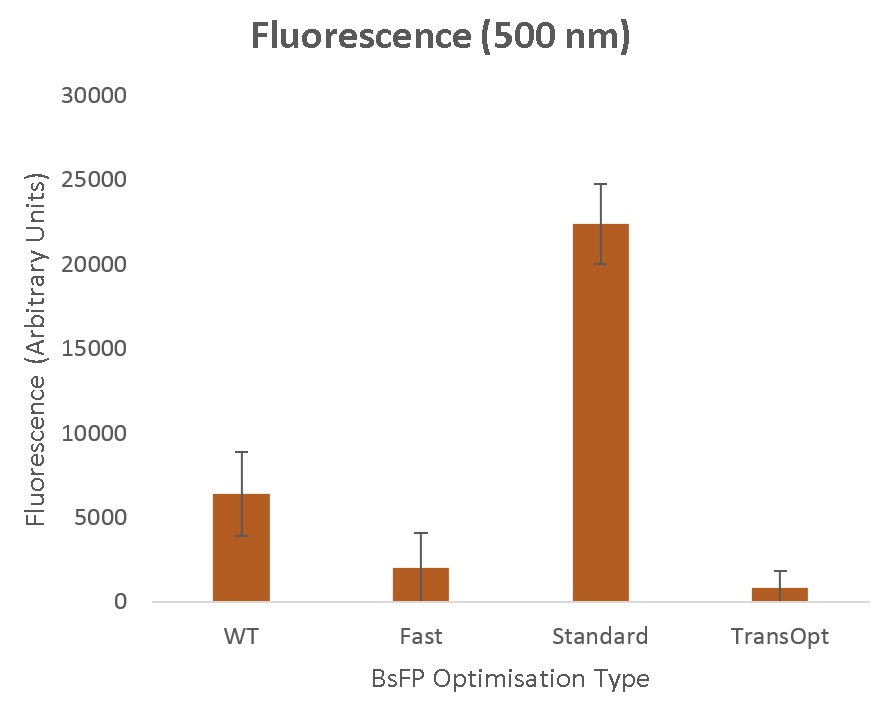
- Fluorescence assay of BsFP showed that protein from standard harmonised algorithm had higher fluorescence while the TransOpt generated one showed no fluorescence, indicating future work is required on the TransOpt algorithm
What did we find?
- Unable to make functional heterologous expression of EtnABCD in P.putida
- Validation of LacI and Q15 constitutive promoter functionality in regulating the lac operon
- TransOpt did not successfully optimise heterologous translation and folding, whilst standard harmonisation did
Full Results
... for the keen beans out there.I. LacI Characterisation
Experiment
LacI-Q15 consisting of LacI represser gene (from the lac operon system) and Q15 promoter upstream of the gene were PCR amplified from the pET15B vector using primers bearing cut sites including SalI, EcoRI and PstI for ligation into pUS44 and pSB1C3 vectors. After PCR clean up using Qiagen PCR kit, we performed digestion and ligation (as prescribed in the protocols page) to generate pSB1C3-LacI (pSB-LacI) recombinant vector and transformed it into P. putida KT2440 cells followed by screening using chloramphenicol (Cm25) plates. Then, we set up patch colony plates and performed colony PCR screening using Taq polymerase to pick colonies bearing the correct pSB-LacI vectors. To further clarify the PCR results, we also performed a single digest to infer the presence of LacI in the final vector by its total size.
Characterisation
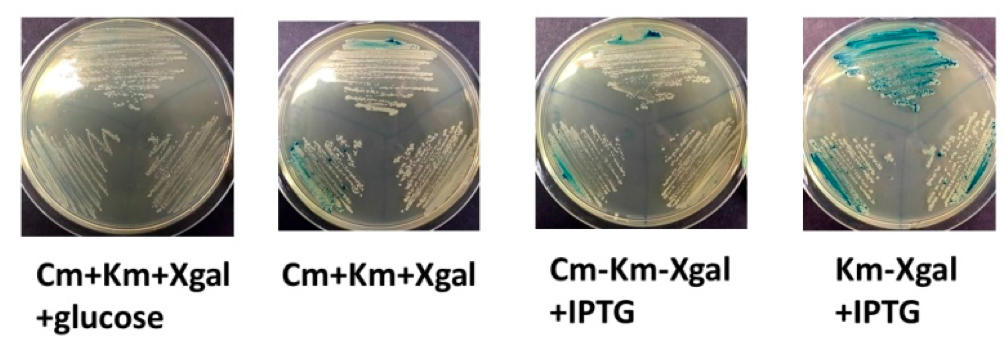
To characterise the LacI repressor gene, we made E.coli JM109 competent cells with the pBBRMCS2 vector containing the LacZ gene (usually as a selective marker). Then, we transformed the cells with LacI-pSB and incubated them over Cm25-Kn50-Xgal50 plates with only one containing IPTG and the other containing no IPTG. After incubation at 37 oC, most colonies on the IPTG plate turned green which indicates Xgal hydrolysis due to LacZ expression as a result of lac promoter activation by IPTG (caused by repressor protein inhibition). At the same time, the colonies on the IPTG deficient plates remained white, which infers the lac of LacZ inhibition due to repression by LacI protein. Hence, the activity of LacI was characterised by taking advantage of the lac operon system from pBBRMCS2.
Purpose
Due to failure of initial NBP assays for EtnABCD activity in P.putida, and lack of growth in pBBRMCS2-EtnABCD transformed JM109 cells, we believed that the presence of a mechanism to control the expression of EtnABCD is important for optimal activity in hosts. In other words, we thought that it would be important to have an added level of control. Hence, this was a supplementary to the EtnABCD project and due to the small size and convenient design of the gene, we evaluated that the development and characterisation of the construct would be quick and simple using straightforward digestion and ligation cloning methods.
II. GroEL-GroES
Experiment
GroEL-GroES (GroES) experimental procedure evolved into two different stages of differing approach. This is due to the fact that results obtained from phase I were not satisfactory and necessitated the design of a second approach.
Phase I
We started by PCR amplifying the two Glocks containing P.putida GroES open reading frames (ORF). After several attempts, we managed to amplify the Gblocks and used them in Golden Gate cloning reaction with XbaI/PstI digested PCR linearised pSB1C3 vector (PCR linearised pSB1C3 primers were designed so as not to amplify the RFP). To screen for successful clones, we transformed into E.coli JM109 cells over agar plates containing chloramphenicol (Cm). After an overnight incubation, the colonies were patched and screened via PCR colony with primer designed to amplify the GroES-pSB1C3 (Gro-pSB) junctions. The clones showing the correct bands for the junctions were grown, and after miniprep, digested with EcoRI single digest and a PstI/EcoRI double digest to check the presence of the correct insert in the recombinant vector by their size after running on agarose electrophoresis. Unfortunately, the digestion analysis gave negative results for all clones, with unexpected band sizes uncharacteristic of the anticipated 2 kbp of GroES. A different approach was taken.
Phase II
The absence of success in phase I contrived us to try a 'plan B'. In this phase, we amplified the E.coli GroES gene from its chromosomal DNA by PCR, with the primer containing the appropriate cut sites (XbaI/SpeI) for cloning into pUCP24 vector (note: this E.coli GroES cannot be submitted as a part hence it was not cloned into pSB1C3). Then, using a simple digestion and ligation reaction, pUCP24-GroES (pUC-Gro) recombinant vector was constructed and screened via transformation into JM109 cells in media containing gentamicin (Gm). Clones were obtained, however, due to change in the course of project approach and design, this construct was not further analysed to confirm correct ligation.
Characterisation
Unfortunately due to the nature of results, lack of time, and change in the overall method design of the project, GroES was not used and characterised. However, it should be tried in future to add to the volume of literature debating the idea that GroES co-expression in different clones enhances expression and activity of enzymes.
Purpose
As mentioned briefly in the project description page, due to reports in the literature that considering the function of GroES, their co-expression along with a major enzyme or protein of interest can help folding into their native conformation. Due to low ethene oxidation activity of P.putida containing the wild-type EtnABCD in the previous work, we believed that GroES might help with the folding and help increase its activity with the assumption being that the low activity was due to incomplete folding into native conformation. The decistion to choose E.coli GroES rather than the initial P.putida was due to reports that the former also functions well in P.putida. Hence, the decision was made to generate E.coli GroES so that it can also be used later in E.coli with EtnABCD, which is the ultimate end goal of this project.
III. EtnABCD Characterisation
This is the main part of our experiment.
Experiment
In this phase, we addressed the problems in the previous work and GroEL-GroES through changing the following in our method:
- Generation of a new harmonised EtnABCD sequence using TransOpt algorithm appropriate and optimised for expression in P.putida
- Co-expression of LacI repressor protein to control the expression of ethene MO as it is under the control of lac promoter
After running the Golden Gate reaction containing the three Gblocks and EcoRI/PstI digested PCR linearised pSB1C3, followed by transformation into JM109 E.coli and selection on Cm selection plates, we screened the colonies by inoculating them in LB with Cm, performing miniprep and digestion analysis. The two digestion reactions were conducted with EcoRI and EcorI/PstI followed by agarose gel electrophoresis. The clones showing the right bands (one large band and two bands in each reaction, respectively) were used to construct pBBRMCS2-EtnABCD (pBBR-Etn) recombinant vectors. The pSB1C3-EtnABCD (pSB-Etn) vector was digested with EcoRI/PstI and ligated with digested pBBR vector. After transformation into P.putida KT2440 strain by electroporation and screening over kanamycin (Kan) plates, the clones were screened via colony PCR. The clones showing results that inferred the presence of Etn in pBBR vector were used ( Need to go over this with Mark) for making KT2440 competent cells. The competent KT2440 cells were incubated to express ethene MO (constitutively due to the 'leakiness' of lac promoter and exposed to ethene to check for activity by screening for the presence of ethane oxide. The screening is performed via NBP assay which turns purple upon the presence of ethane oxide.
To add the extra level of control of ethene MO expression, the co-expression of LacI alongside ethene MO was also performed.
Characterisation
Purpose
The introduction of LacI co-expression into the ethene MO expression system controlled by the lac operon was a later addition to the experimental design. The reason for this late adjustment was the hypothesis after the first unsuccessful NBP assay that possibly the overexpression and lack of control over the expression of ethene MO may be toxic to the cells. To add that layer of control, LacI repressor protein was co-expressed to control the ethene MO expression by exploiting lac operon. This hypothesis was also supported by our observation that pBBR-Etn transformed e.coli cells were unable to grow well despite confidence in the presence of the plasmid and reference to the controls. While it is justified to say that this could be due to the fact that ethene MO is not harmonised (or well-suited) for e.coli expression, it may be equally likely that the retardation of cell growth is due to the hostility of constitutive or overexpression of ethene MO in e.coli. This suggests that this may be the same reason that ethene MO is not functional in p.putida.
The motivation for the use of Golden Gate cloning method is outlined in the Golden Gate information page. Furthermore, the use of weaker ribosomal binding sites (RBS) upstream of each ORF and harmonisation of ethene MO sequence all aid the efficient translation and folding of the enzyme into its native conformation. Codon harmonisation ensures that the ribosome translation kinetics over ethene MO are conserved, as it affects protein folding, while weaker RBS ensures the inhibition of ribosomal traffic on the mRNA which can adversely affect mRNA translation and protein folding.
TransOpt: Experimental Validation
In order to assess the performance of our novel codon optimisation algorithm we performed an experimental test to investigate its capability to generate a functional mRNA sequence that yields well-folded and highly functional proteins. Though we used this same algorithm to generate an optimised sequence for ethene MO in the target host p.putida (to preserve the translational kinetics profile of the ethene MO mRNA between the native and heterologous host), we saw it appropriate to perform a more simple and targeted validation test, via which we could assess other assumptions made in our work. This is because ethene MO would be too complex a protein for us to (on a single-case basis) reliably analyse and draw conclusions regarding the performance of the algorithm, as there are too great a number of contributing variables to the activity of the enzyme. Hence, we decided to use fluorescent protein as the target of our algorithm. Fluorescent proteins, such as GFP, are used widely in the literature as proteins to test the viability of codon optimisation algorithms (as discussed on the TransOpt page), with the major assumption being that the level of fluorescence is proportional to the efficacy of protein production and folding into the native conformation.
In our study, the b. subtillis flavin-binding fluorescent protein (BsFP) was used as a marker to test the validity of the different methods for codon optimisation. The particular protein (BsFP) is a promising new alternative to the green fluorescent protein (GFP) family of proteins: BsFP is a small protein (137 amino acids), it is functional in many different cellular environments and has high tolerance to changes in pH, oxygen levels and heat, making it applicable to a wide range of experiments. In contrast, GFP is much larger, matures slowly, absolutely requires oxygen for fluorescence, and is sensitive to chemical changes 1. Furthermore, in the context of our planned experiments, the lack of genome sequence for the GFP source organism aequorea victoria means that no tRNA GCN profile is available, which is crucial for the newly-developed harmonisation algorithm. However, the choice of this fluorescent protein does present downsides: It may not include complex folding domains, the presence of which motivated TransOpt's designed capability for regulating translation rate trends. Furthermore, fluorescent markers were the prototypical proteins upon which many of the existing codon optimisation methods have been developed, and so it would not be surprising if standard optimisation methods succeed here, though they may fail on more complex sequences (see Scientific Background of TransOpt). Thus, without a complex folding structure, the benefits afforded by TransOpt might be minimal (for example, in comparison to standard codon harmonisation algorithms).
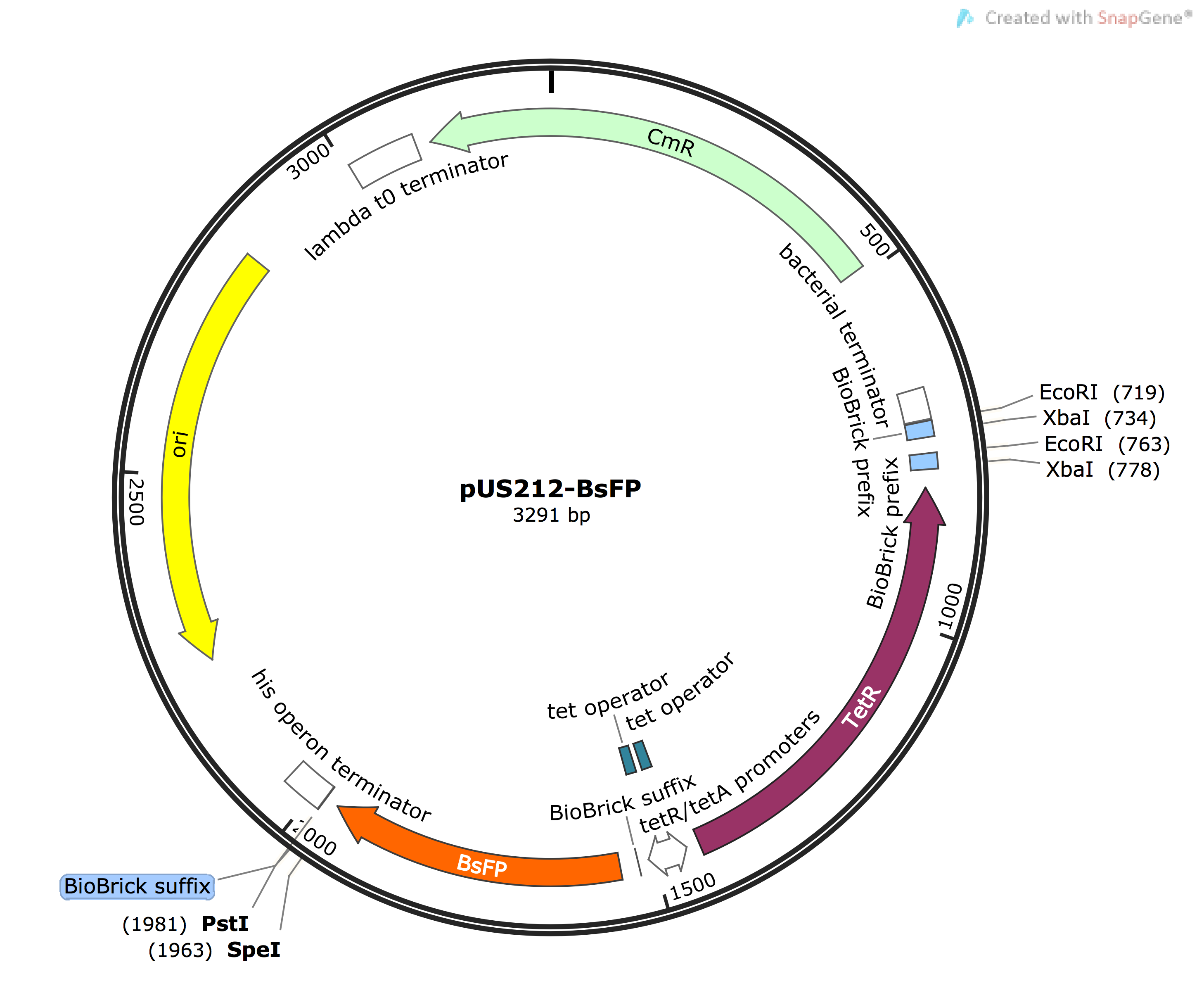
The following four BsFP sequences were generated, cloned in to pUS212 vector and expressed in tetracycline (TetR) - in low amounts that do not exhibit antibiotic properties - and induced in e.coli to verify the algorithm. We generated all sequences ourselves, as described on the TransOpt page, using a rate quantisation methodology based on the organisms' tRNA Gene Copy Numbers.
- BsFP-WT: native sequence from the native host b.subtilis
- BsFP-fast: all codons replaced with their synonyms possessing the highest rate
- BsFP-standard: harmonised BsFP generated via standard harmonisation
- BsFP-TransOpt: optimised sequence generated using the TransOpt algorithm
By measuring changes in fluorescence, the folding of the protein can be determined. We expect that high fluorescence will be detected from the standard harmonised variant (as demonstrated by past studies), but we hope that the TransOpt variant's fluorescence will be better still, due to superior folding than the other variants. We expect the fast optimised sequence will demonstrate a decreased fluorescence compared to the wild type, as these rate-maximisation approaches to optimisation have been shown to be flawed in past studies (see discussion on the TransOpt page).
Method
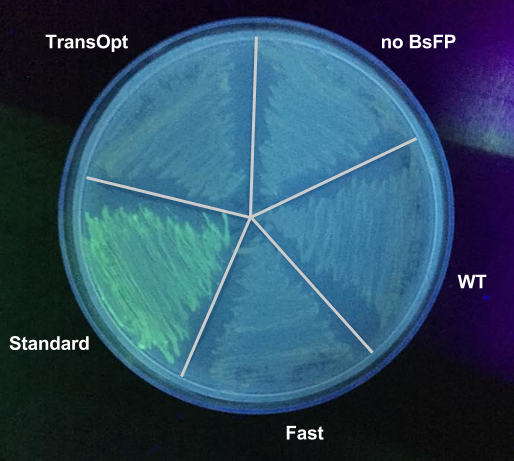
All four BsFP GBlocks were designed to have the EcoRI/XbaI and SpeI/PstI cut sites at either end of the ORF. Firstly, BsFP was cloned into pUS212 expression vector via digestion with XbaI and PstI followed by ligation. To screen for the recombinant vector, the ligation mix was transformed into JM109 e.coli and screened over agar LB plates containing chloramphenicol (Cm). Then, the colonies were used for colony PCR to check for the presence of the ligated insert and subsequently patched on a new plate with TetR (100 ng/uL) to induce BsFP expression. To gain an immediate qualitative data of the induced patch colonies, they were exposed to UV light and screened for presence of green fluorescent colonies. Using this knowledge and the PCR colony results after running agarose gel electrophoresis, an equal number of appropriate colonies of each BsFP variant was picked, inoculated, and induced in LB broth containing Cm and TetR (TetR is added after the culture reaches the appropriate OD600. Finally, four hours after the addition of the inducer, the samples were taken and had their OD600 and fluorescence measured with excitation at 460 nm and emission at 500-520 nm, with controls such as GFP and empty pUS212 vector containing no BsFP gene. To normalise the data, the fluorescence arbitrary unit of the empty pUS212 containing colonies was subtracted from each BsFP variant.
To develop the pSB1C3-BsFP recombinant vector, BsFP Gblock was digested with EcoRI/PstI, ligated, transformed and screened by exposure to Cm, performing colony PCR and sequencing.
Results Summary
Our raw results are in the figure below, demonstrating that the standard codon harmonisation method and the fast-codon optimised method performed as expected when compared to the wild type. However, the TransOpt method did not function as desired, producing levels of fluorescence indistinguishable (to within experimental error margins) from those in the control case. Aside from the distinct possibility of errors in the experimental procedure used to prepare this variant for measurement, a number of sequence-based causes may have been responsible for this negative result, as discussed on the TransOpt Experimental Validation page.
In conclusion, we were able to achieve the following aims:
- Demonstrated that the sequence generated via the standard codon harmonisation algorithm was able to give improved fluorescence, supporting the rate quantisation methodology we have employed.
- Demonstrated that the sequence generated via selecting fast-translating codons results in decreased fluorescence, supporting our hypothesis that the use of only fast-translating codons may result in slowed (and possibly stalled) translation.
However, we came short of reaching the following aim:
- Demonstrate that TransOpt improves upon the standard codon harmonisation method.
For full analysis of these results, discussion of their implications for the effectiveness of our algorithm, and identification of potential avenues for future improvement, please see the TransOpt page.
References
1 Mukherjee, A., et al., Characterization of flavin-based fluorescent proteins: an emerging class of fluorescent reporters. PLoS One, 2013. 8(5): p. e64753.