Team:Austin UTexas/Project/Plasmid Study
Measuring Fluorescent Protein Gene Stability
Introduction
Methods
Plasmid Construction
Plasmids were constructed with standard BioBrick cloning procedures and enzymes. Composite BioBricks were constructed using the following unique BioBrick combinations:
| Plasmid | Fluorescent Protein BioBrick | Fluorescent Protein | Promoter/RBS BioBrick | Promoter;RBS Strength |
| 1 | BBa_K592100 | BFP | BBa_K608004 | Strong; Weak |
| 2 | BBa_K592100 | BFP | BBa_K608007 | Medium; Weak |
| 3 | BBa_E0020 | ECFP | BBa_K608007 | Medium; Weak |
| 4 | BBa_E0020 | ECFP | BBa_K608003 | Strong; Medium |
| 5 | BBa_E0020 | ECFP | BBa_K608002 | Strong; Strong |
| 6 | BBa_K592101 | YFP | BBa_K608002 | Strong; Strong |
| 7 | BBa_K592101 | YFP | BBa_K608006 | Medium; Medium |
| 8 | BBa_K592101 | YFP | BBa_K608007 | Medium; Weak |
| 9 | BBa_K864100 | SYFP2 | BBa_K608002 | Strong; Strong |
| 10 | BBa_K864100 | SYFP2 | BBa_K314100 | Strong; Very Strong |
| 11 | BBa_K864100 | SYFP2 | BBa_K608006 | Medium; Medium |
| 12 | BBa_E0030 | EYFP | BBa_K608007 | Medium; Weak |
| 13 | BBa_EE030 | EYFP | BBa_K608006 | Medium; Medium |
| 14 | BBa_E0030 | EYFP | BBa_K314100 | Strong; Very Strong |
Several fluorescent protein/promoter+RBS combinations were studied in more than one experiment, which is why each plasmid above is classified as a 'unique' combination.
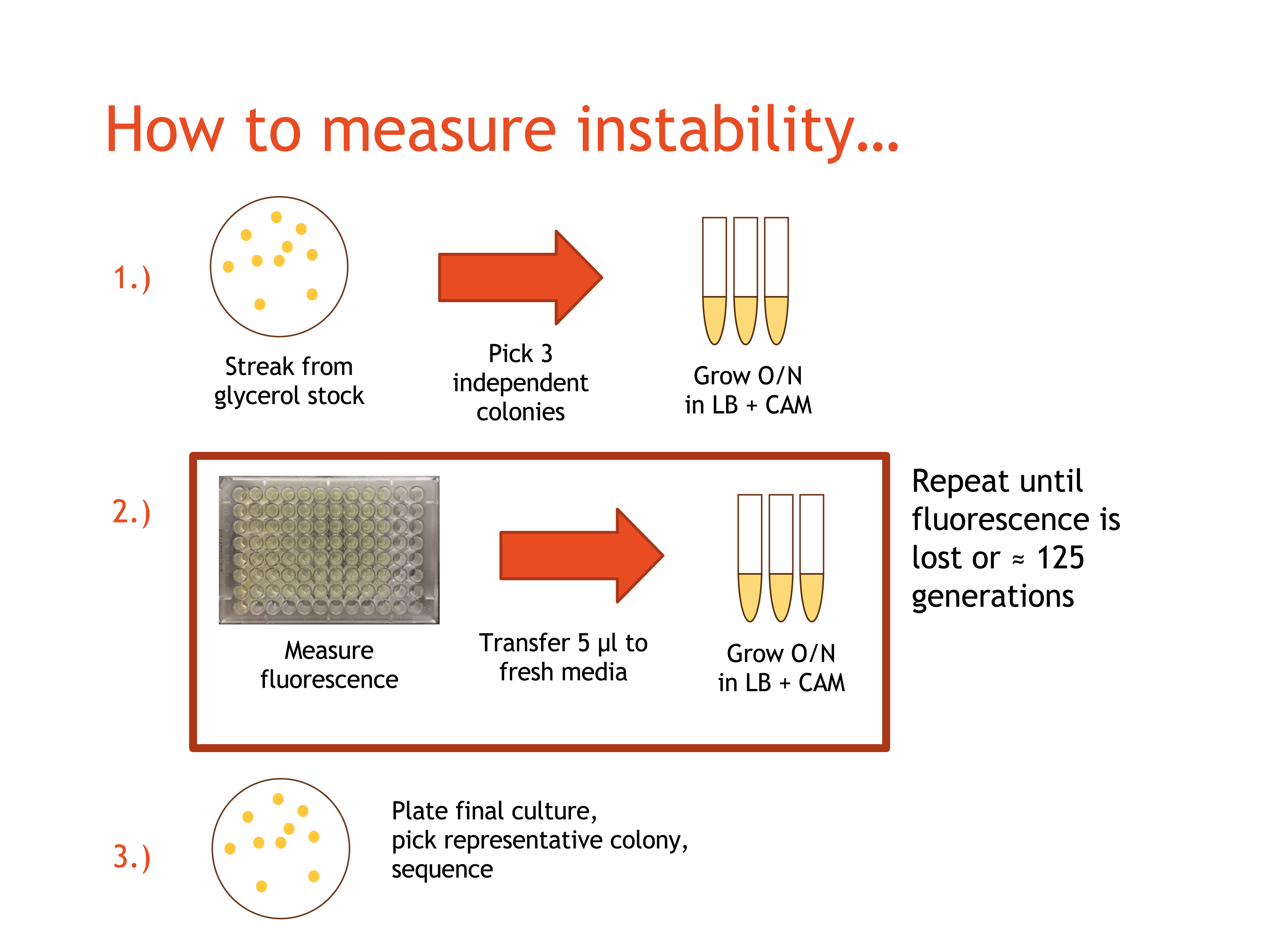
The BioBricks in each pair were ligated together using T4 DNA Ligase, and transformed into TOP10 E. coli. Prepared cultures were streaked onto LB/chloramphenicol (CAM) agar plates, and were placed in a 37°C incubator overnight. After at least 20 hours, the plates were retrieved, and from each plate three independent, fluorescent colonies were selected and each placed into 5mL of LB/CAM media. The tubes for each were labelled with the date, the culture name, DAY 1, and the strain used. The tubes were placed in a 37°C shaker-incubator and the plates were placed in a 4°C cold room for storage.
Fluorescence Readings
The primary aim of this project was to assess the fluorescent protein coding sequences that are most prone to breaking/mutating. In order to do this, we had to propagate different fluorescent proteins and record their fluorescence daily.
The day immediately following the start of the DAY 1 cultures, the DAY 1 cultures were retrieved and resuspendeded. Three empty culture tubes for each participating team member were collected and labelled the same way as the first set of culture tubes, but with DAY 2 instead of DAY 1. 5mL of LB/CAM media were placed into each culture tube, and 5μL of culture from the microcentrifuge tubes were pipetted into the appropriate culture tube. The culture tubes for DAY 2 were placed into the shaker-incubator, and the DAY 1 culture tubes were placed in the cold room.
200μL of each DAY 1 culture were pipetted into a well on a 96-well plate (which was stored on ice when not in use). The well number, date, and culture name were recorded, and the plates were read for fluorescence the following morning. This process of moving cultures forward and obtaining fluorescence readings was repeated daily until the culture fluorescence dropped dramatically, or until DAY 10, so that each culture had a maximum 10 culture tubes over the 10 days.
The DAY 10 cultures were allowed to incubate and were sent for sequencing. A full "control" plasmid sequence was created for each culture by inserting the recorded BioBrick sequences into an existing pSB1C3-circular sequence on Benchling.com. The sequencing results were uploaded to Benchling and compared to the predicted original sequence of the plasmid for any possible mutations.
summary table or figure(?)
Spring 2015 Data and Observations<--This section and title need to be revised [notice the "question marks" below.</b>
Data from the first experiment gave a strong indication as to whether or not a coding sequence was stable. Specifically, plasmids containing coding sequences for BFP, SYFP2 and YFP broke swiftly while coding sequences for EYFP and ECFP remained mostly intact by the experiment’s conclusion.
Several types of mutations were observed: transposon/IS element insertions*, point mutations, various deletions, and phage insertions. IS element insertions were the most common mutation, and occurred exclusively in BFP, SYFP2 and YFP, suggesting that these plasmids are less stable than the others. 23 of 40 independent cultures for these coding sequences exhibited a loss of fluorescence due to transposon element insertions.
The next most common mutation were single nucleotide point mutations, with 6 point mutations observed. Four of these point mutations occurred in a promoter, which could One was located in an RBS (which?) and one altered a start codon. Further, five samples incurred promoter deletions (which promoters?), and five additional samples developed miscellaneous large and small deletions. Finally, three samples with coding sequences for BFP contained the same phage element, suggesting that this event occurred before independent colonies were selected. (possibly add some discussion about promoters—repeat mediated?)
Our results displayed an array of mutations, and potential experiments for follow up. We decided to focus on IS element insertions, which were most prevalent. These insertion elements are found in the genome of Top10 E. coli. They replicate ~independently*? and insert themselves into different DNA locations. If this DNA location happens to be within the inserted plasmid, the genetic device breaks, granting a depreciated metabolic load in the mutant and reducing fluorescence. A number of mobile elements in E. coli have been identified. In particular, we observed mobile elements IS10L, IS10R and IS1, with the latter occurring only once*. Interestingly, in plasmids with YFP and BFP, these IS elements exhibited only moderate insertion preference. In these sequences, IS elements were found throughout both promoters and coding sequences. However, in SYFP2 plasmids, IS10L/R expressed preference for a particular nucleotide location (GGCGTAGTACC) near the start of the SYFP2 plasmid. (same nucleotide sequence in each plasmid?)* Based on this preliminary data, we consider coding sequences for BFP, SYFP2 and YFP to be unstable while we consider coding sequences for EYFP and ECFP to be stable. Consequently, we decided to focus on SYFP2 as we continued studying instability.
(WILL BE MOVED TO FIRST DATA SET)
Results
Plasmid Stability
insert mutations in SFYFP
TOP-10 E. coli was transformed using various fluorescent genes and monitored for breakage, with varying results depending on the plasmid used. Three plasmids were selected from the spring experiment to be used. All three plasmids used the pSB1C3 backbone and the vector BioBrick BBa_K608006, composed of a medium promoter (J23110) and medium ribosome binding site (B0032). Each plasmid then differed by the fluorescent protein used: Yellow fluorescent protein (BBa_K592101), Super-folder Yellow fluorescent protein (BBa_K864100), and Engineered Yellow fluorescence protein (BBa_E0030). The YFP plasmid was to shown, during the spring, was shown to accumulate various insertions and deletions in the YFP gene that would lead to its breakage. The Super-folder YFP plasmid was broken by a large insertion after the promoter sequence that led the breakdown of expression. Finally, the engineered-YFP gene, which was codon optimized from the original YFP sequence, did not accumulate mutations or break over the course of ten days.
EYFP, CYFP stable
Mutations
what contributes to stability
what does this mean about what sequences are stable
next steps