Difference between revisions of "Team:UCLA/Notebook/Honeybee Silk/8 July 2015"
| (2 intermediate revisions by the same user not shown) | |||
| Line 289: | Line 289: | ||
='''Inoculation of 5/19 Transformation'''= | ='''Inoculation of 5/19 Transformation'''= | ||
| − | Recent experiments have proven that | + | Recent experiments have proven that the pET24a miniprepped sample was not correct. Today I will repick colonies and grow them up for miniprepping. |
I picked two colonies from the 1:1 kanamycin plate and suspended them in 100uL ddH2O. Each sample was incubated at 37C with 5mL LB broth and 5uL 1000X kanamycin. | I picked two colonies from the 1:1 kanamycin plate and suspended them in 100uL ddH2O. Each sample was incubated at 37C with 5mL LB broth and 5uL 1000X kanamycin. | ||
| − | |||
='''PCR of Honeybee G-block and Purification'''= | ='''PCR of Honeybee G-block and Purification'''= | ||
| Line 399: | Line 398: | ||
! Incubation | ! Incubation | ||
| 37C | | 37C | ||
| − | | 1 | + | | 1 hour |
|- | |- | ||
! Heat Inactivation | ! Heat Inactivation | ||
| Line 406: | Line 405: | ||
|- | |- | ||
!Hold | !Hold | ||
| − | | | + | | 10C |
| Hold | | Hold | ||
|} | |} | ||
| Line 413: | Line 412: | ||
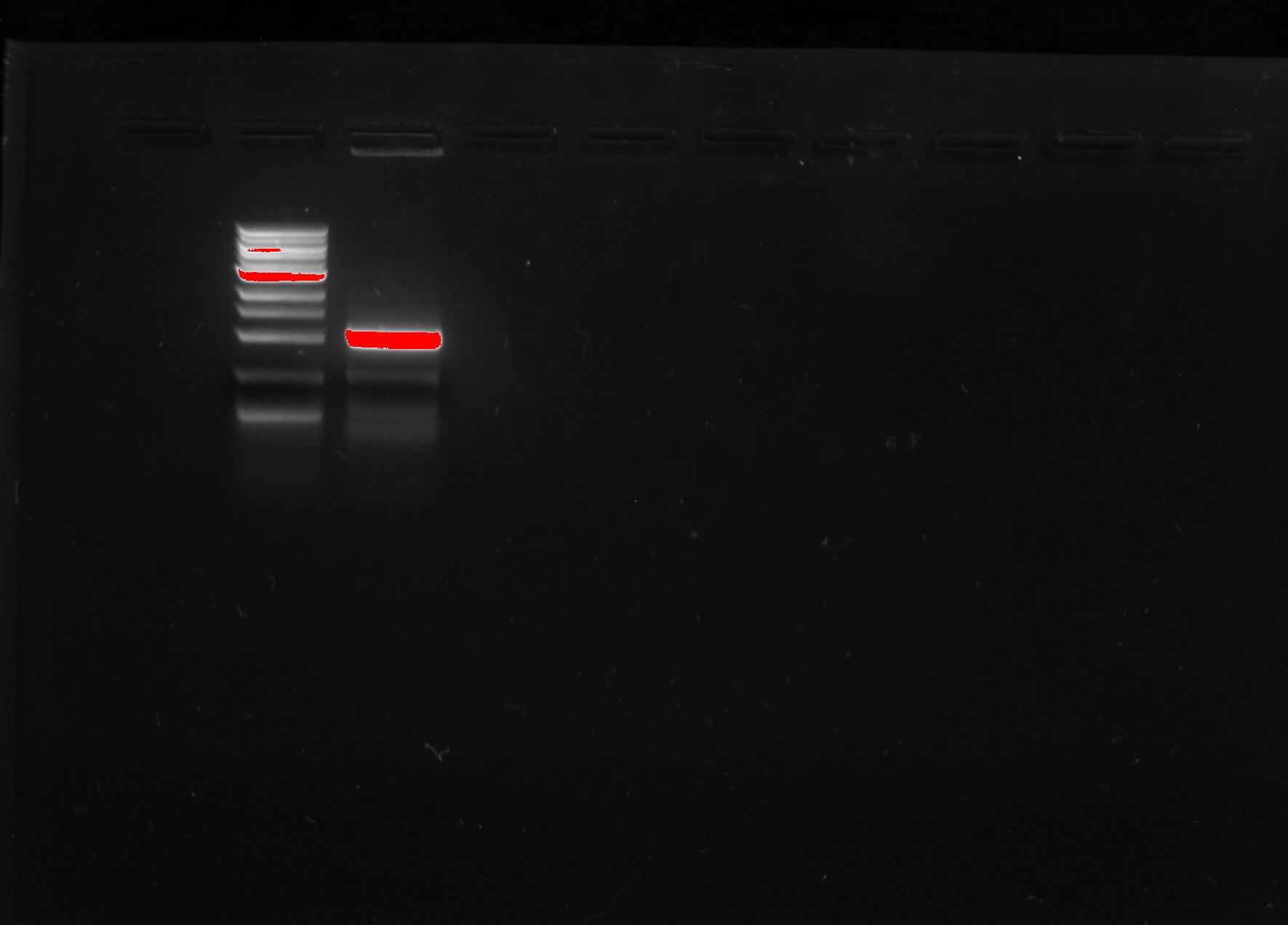
The sample was loaded onto a 1% agarose gel with 10uL 6X loading dye against a 1kB ladder. | The sample was loaded onto a 1% agarose gel with 10uL 6X loading dye against a 1kB ladder. | ||
Expected band size: ~1000bp | Expected band size: ~1000bp | ||
| − | |||
| + | Results: | ||
| − | + | [[File:Gel 2015-07-08.jpg|none|thumb|500px|Gel image]] | |
Latest revision as of 20:14, 15 July 2015
Contents
Inoculation of 5/19 Transformation
Recent experiments have proven that the pET24a miniprepped sample was not correct. Today I will repick colonies and grow them up for miniprepping.
I picked two colonies from the 1:1 kanamycin plate and suspended them in 100uL ddH2O. Each sample was incubated at 37C with 5mL LB broth and 5uL 1000X kanamycin.
PCR of Honeybee G-block and Purification
The concentration obtained after digestion and purification was too low (20.12ng/uL), today I will be redoing the PCR, digestion, and purification to obtain a higher yield.
| Component | Volume (out of 50uL) |
|---|---|
| 5X Q5 Reaction Buffer | 10uL |
| 10mM dNTPS | 1uL |
| 10mM primer 10 | 2.5uL |
| 10mM primer 11 | 2.5uL |
| Template (Honeybee G-block) | 1uL |
| Q5 High Fidelity DNA Polymerase | 0.5uL |
| Nuclease Free Water | 32.5uL |
Program
| Step | Temperature | Time |
|---|---|---|
| Initial Denaturation | 98C | 30s |
| Cycles (x25) | 98C | 10s |
| Annealing | 67C | 20s |
| Extension | 72C | 30s |
| Final Extension | 72C | 2min |
| Hold | 12C | Hold |
The sample was then purified using the Zymo DNA Concentrate and Cleanup Kit. OD: 283.09ng/uL
Digestion and Purification
| Component | Volume (out of 50uL) |
|---|---|
| NEB 2.1 Buffer | 5uL |
| BamHI | 1uL |
| NdeI | 1uL |
| PCR Purified Honeybee G-block | 5uL |
| Nuclease Free Water | 38uL |
Program
| Step | Temperature | Time |
|---|---|---|
| Incubation | 37C | 1 hour |
| Heat Inactivation | 80C | 15 min |
| Hold | 10C | Hold |
The sample was loaded onto a 1% agarose gel with 10uL 6X loading dye against a 1kB ladder.
Expected band size: ~1000bp
Results: