Difference between revisions of "Team:Czech Republic/Microfluidics"
(→References) |
(→Bonding of PDMS to the glass substrate) |
||
| Line 23: | Line 23: | ||
[[File:Microfluidic_chips.jpg|thumbnail|Fabricated microfluidic devices (three PDMS molds bonded to single glass slide)]] | [[File:Microfluidic_chips.jpg|thumbnail|Fabricated microfluidic devices (three PDMS molds bonded to single glass slide)]] | ||
| − | Prepared PDMS replicas with imprinted micro-structures are cleaned properly with scotch tape. The PDMS and glass substrate are treated by oxygen plasma for 20 seconds. The oxygen plasma affects the PDMS backbone and forms reactive silanol functional groups (Si-OH) enabling formation of permanent irreversible covalent bond of the PDMS to the glass substrate {{:Team:Czech_Republic/Template:ReferenceRef|Wong2009}}. In addition, the PDMS treatment with oxygen plasma is ben- eficial as it avoids nonspecific adsorption, decreases cell clogging and turns the PDMS to hydrophilic. The hydrophilicity of PDMS facilitates the future microchannel wetting | + | Prepared PDMS replicas with imprinted micro-structures are cleaned properly with scotch tape. The PDMS and glass substrate are treated by oxygen plasma for 20 seconds. The oxygen plasma affects the PDMS backbone and forms reactive silanol functional groups (Si-OH) enabling formation of permanent irreversible covalent bond of the PDMS to the glass substrate {{:Team:Czech_Republic/Template:ReferenceRef|Wong2009}}. In addition, the PDMS treatment with oxygen plasma is ben- eficial as it avoids nonspecific adsorption, decreases cell clogging and turns the PDMS to hydrophilic. The hydrophilicity of PDMS facilitates the future microchannel wetting {{:Team:Czech_Republic/Template:ReferenceRef|Kalio2006}}. Immediately after the oxygen plasma treatment, small droplet of methanol is poured over the glass substrate to avoid instantaneous bonding of the activated PDMS to the glass substrate. The methanol between the glass and the PDMS increases the time necessary for alignment of the PDMS micro-structures above the patterned micro-electrode topologies. The alignment is performed manually under binocular. The glass substrate bonded to PDMS is placed in the oven, for 5 minutes at 80°C. The bonded devices were stored in a room temperature. Inlets and outlets were sealed with scotch tape to avoid contamination. |
= Experimental setup = | = Experimental setup = | ||
Revision as of 15:03, 10 September 2015
Microfluidics
Contents
Introduction
The diffusion processes are slow, and the inertial effects are negligible on micro-scale with low Reynolds number [Angelescu2011] [Nam2002]. Hence microfluidics became a valuable tool enabling complex control of the cellular microenvironment. Microfluidic experiments in conjunction with live fluorescence microscopy were designed and performed to verify and characterise the developed IOD system. Microfluidic devices were fabricated by standard PDMS soft-lithography technology.
Soft-lithography
Microfluidic channels were formed using PDMS soft-lithography technology, which has proven biocompatibility and can be readily applied in available laminar flow cabinets. Photomask and silicon master fabrication was outsourced. Fabrication of microfluidic devices was divided in two subsequent steps. In the first step, silicon masters were used for the PDMS molding. In the second step, PDMS molds were bonded to the glass substrates to form encapsulated microfluidic devices using air plasma technology.
PDMS molding
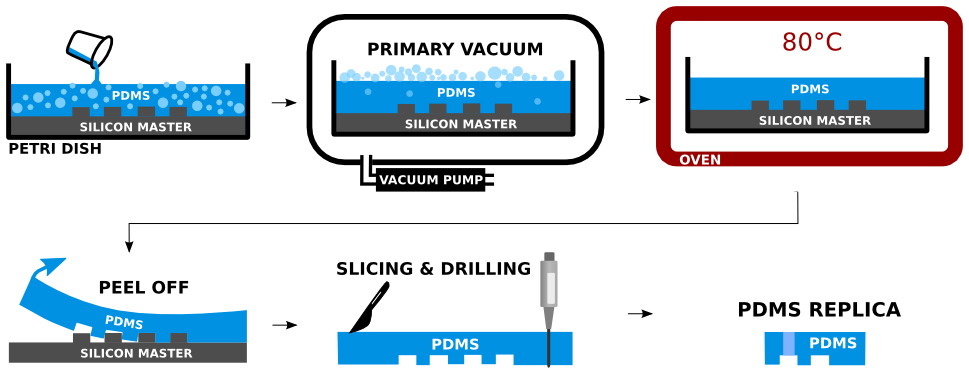
Two part silicone elastomer Sylgard 184 is used to produce PDMS. The base part is mixed with sufficient amount of curing agent (10:1 ratio) and stirred well in a disposable plastic cup. The mixture is placed in dessicator to remove the air bubbles introduced by the mixing. The Si master is placed in a petri dish and the mixture is poured over. The remaining air bubbles are removed from the PDMS by sharp tip of a needle. The poured PDMS is maintained in perfect horizontal position to assure good planarity, and is cured in an oven, for 2 hours at 80°C. The PDMS edges are cut off with sharp tool and the PDMS is peeled off the Si master. The PDMS mold is sliced into sections containing individual devices. Inlets and outlets are drilled carefully by biopsy punch of the appropriate diameter at the desired locations of the PDMS replica.
Bonding of PDMS to the glass substrate
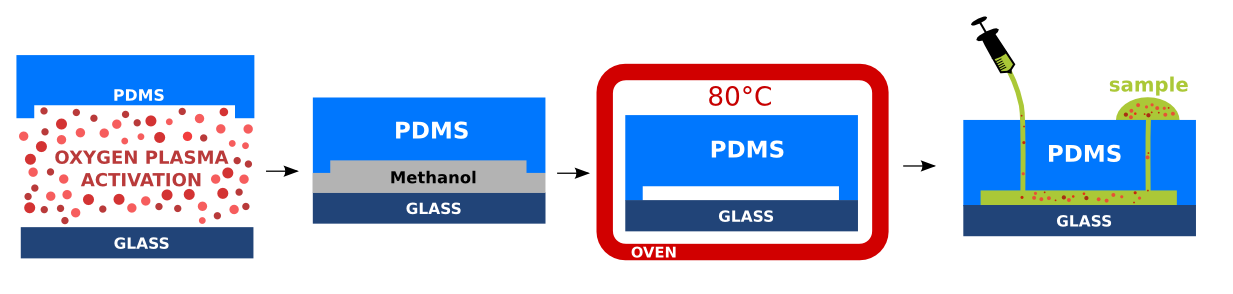
Prepared PDMS replicas with imprinted micro-structures are cleaned properly with scotch tape. The PDMS and glass substrate are treated by oxygen plasma for 20 seconds. The oxygen plasma affects the PDMS backbone and forms reactive silanol functional groups (Si-OH) enabling formation of permanent irreversible covalent bond of the PDMS to the glass substrate [Wong2009]. In addition, the PDMS treatment with oxygen plasma is ben- eficial as it avoids nonspecific adsorption, decreases cell clogging and turns the PDMS to hydrophilic. The hydrophilicity of PDMS facilitates the future microchannel wetting [Kalio2006]. Immediately after the oxygen plasma treatment, small droplet of methanol is poured over the glass substrate to avoid instantaneous bonding of the activated PDMS to the glass substrate. The methanol between the glass and the PDMS increases the time necessary for alignment of the PDMS micro-structures above the patterned micro-electrode topologies. The alignment is performed manually under binocular. The glass substrate bonded to PDMS is placed in the oven, for 5 minutes at 80°C. The bonded devices were stored in a room temperature. Inlets and outlets were sealed with scotch tape to avoid contamination.
Experimental setup
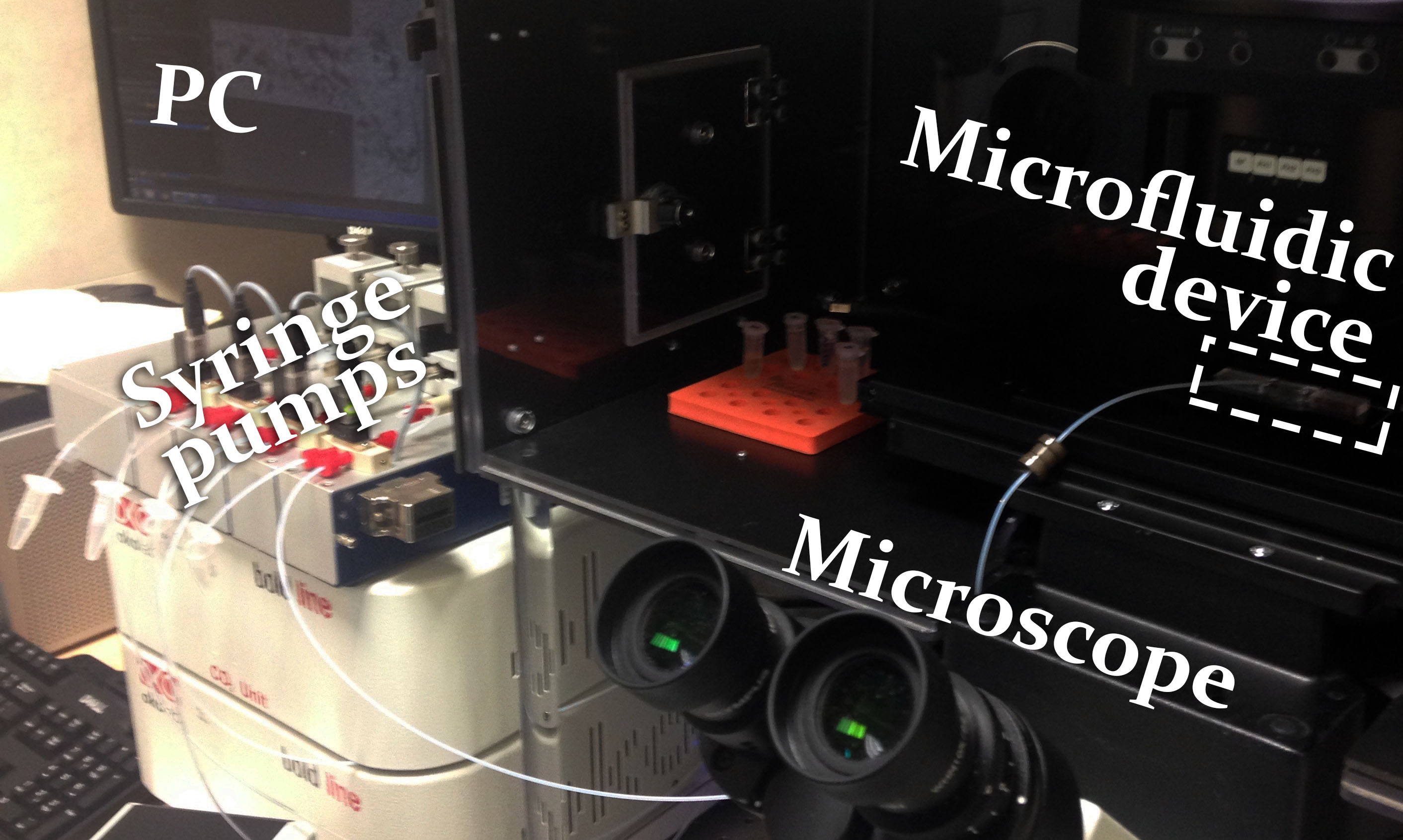
Microfluidic experiments were conducted on a platform already established by the Georgiev lab. The laboratory is equipped with precise microfluidic syringe system (neMESYS Low Pressure Syringe Pumps), and microscopic station enabling fluorescence imaging and live cell microscopy (Olympus IX83).
Microfluidic experiments
Signal transmission distance and dynamics
Agglutination on chip
Personnel
- Martin Cienciala - Microfluidic experiments
- Vaclav Pelisek - Fabrication of microfluidic devices
- Pavel Fikar - Scientific advisor
References
- ↑ Dan E. Angelescu (2011). Highly Integrated Microfluidic Design. Artech House, Norwood.
- ↑ Nam-Trung Nguyen and Steven T Wereley (2002). Fundamentals and applications of microfluidics. Artech House.
- ↑ Ieong Wong (2009). Surface molecular property modifications for poly(dimethylsiloxane) (pdms) based microfluidic devices. Microfluid Nanofluid, 7:291–306.
- ↑ Johana Kuncova-Kallio (2006). Pdms and its suitability for analytical microfluidic devices. In Proceedings of the 28th IEEE EMBS Annual International Conference, New York City, USA, 9, IEEE.