Difference between revisions of "Team:Czech Republic/Project/Orthogonal signals and receptors"
(→Test of orthogonality (fluorescence approach)) |
(→Test of orthogonality (fluorescence approach)) |
||
| Line 306: | Line 306: | ||
Since the orthogonality tests using the growth arrest allowed us to verify orthogonality of only two receptors, we decided to continue these assays using fluorescence as the output. | Since the orthogonality tests using the growth arrest allowed us to verify orthogonality of only two receptors, we decided to continue these assays using fluorescence as the output. | ||
| + | |||
| + | We transformed MATa cells (Δbar1) with the pRSII415_pADH1_Receptor plasmids (to produce and display different receptors on the cell) together with the pFUS1_GFP plasmid (to detect the activation of MAPK by an increase of fluorescence). | ||
<table class="ig2fluor wikitable"> | <table class="ig2fluor wikitable"> | ||
Revision as of 14:58, 18 September 2015
Orthogonal signals and receptors
Contents
Abstract
In order to achieve more complex behaviour of our IODs, each IOD needs to be able to communicate with others. Since the pheromone response pathway was used for signal transduction, we prepared several synthetic signals and receptors that couple to this pathway and allow our IODs to communicate without a cross-talk.
Key Achievements
- Constructed a set of yeast plasmids with different mating pheromones and their receptors.
- Verified the correct coupling of the receptors to the yeast pheromone mating pathway.
- Verified the correct expression and secretion of the different pheromones.
- Contributed to the Registry with 6 BioBricks
Intro / Background
By sensing mating pheromones, S. cerevisiae cells can find their mating partners. We decided to exploit this mechanism for a communication between our IODs.
As was reported in [Janiak2005], it is possible to express chimeric STE2 receptors and couple them to the existing mating pathway. However, since each strain has differences in the G-protein that interacts with the receptor, this coupling is not very efficient. Fortunately, as reported in [Lin2011], this efficiency can be increased by a change of the STE2 C-terminus.
We decided to test five STE2 receptors from different yeast strains. One of them with two different C-termini to see the effects of this change on the signal transduction. We constructed a plasmid expressing the corresponding pheromone for each of these receptors.
Design
Concept
Receptors
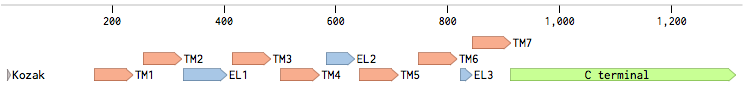
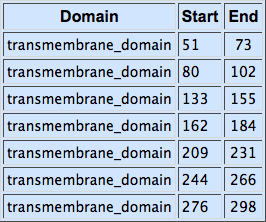
The alpha-factor receptor (STE2) is produced and displayed by MATa cells. It is a 431aa-long seven transmembrane-domain-containing (TM in the figure) GPCR. Since the sequence is known, it can be easily obtained by a PCR from the genome of our Saccharomyces cerevisiae strain. For higher translation in yeast, we added consensus Kozak sequence before the start codon. Also, restriction sites were added to the ends of the fragment to allow for easy cloning.
Other STE2 receptors (STE2 receptors from different yeast strains) were obtained via gBlocks® order. In order to achieve effective coupling to the pheromone mating pathway in the Saccharomyces cerevisiae strain and proper expression, we performed several tasks.
First, after the initial protein [http://blast.ncbi.nlm.nih.gov/Blast.cgi BLAST] search, codon optimization was done using an online tool from IDT. During this process, all BioBrick restriction sites were removed from the DNA sequence.
Second, for each of the STE2 receptor, its C-terminus was replaced by the C-terminus from Candida albicans or Saccharomyces cerevisiae in order to improve coupling to the pheromone mating pathway [Lin2011]. The effect of each replacement on the protein folding was checked using the [http://elm.eu.org/ ELM resource]. Using this tool, we have verified that the changes in the C-termini have no effect on the transmembrane domains.
Finally, the DNA sequences were optimized according to the recommendations from the IDT ordering form to speed up the synthesis process.
Signals
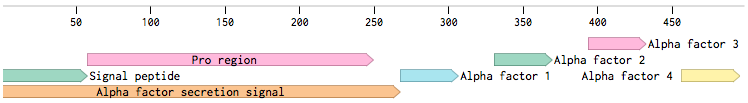
Alpha-factor mating pheromone is a short peptide (typically 13aa long) secreted by alpha cells. For our project, we needed to produce a variety of these pheromones and secrete them from the cells. Although there are some secretion tags present in the registry ([http://parts.igem.org/Part:BBa_K416003 BBa_K416003], [http://parts.igem.org/Part:BBa_K792002 BBa_K792002]), there are no parts encoding pheromones available. Also, since we found evidence that the complete wild-type signal tag is needed for the proper secretion of the pheromone [Caplan1991], we decided to obtain the whole sequence directly from the MF(ALPHA)1 locus.
MF(ALPHA)1 gene codes for four mature alpha-factors within a putative precursor of 165 amino acids. This sequence begins with a signal tag for secretion and a segment with three glycosylation sites (89aa). The second segment contains four mature alpha-factors, each preceded by spacer peptides, which contain proteolytic processing signals [Kurjan1982].
Since we wanted the pheromone peptides to be easily changeable by Site-directed mutagenesis (SDM), we decided to preserve the signal tag with only one copy of the pheromone:
This would create a unique site for the SDM. The final production level of the pheromone is supposed to be practically the same since the used plasmid is present in the cell in several copies (2-5). Also, for higher expression in the cells, we added consensus Kozak sequence before the start codon. Restriction sites were added to the ends of the sequence for easy future cloning.
Materials and methods
Used strains
- E. coli
-
- DH5α
- S. cerevisiae
-
- 7283
- 7284
- Δbar1
- 6193
Used material / chemicals
- LB-M agar plates with ampicillin and chloramphenicol
- 1.5 ml eppendorf tubes
- 0.5 ml PCR tubes
- 50 ml centrifuge conical base and rim tubes
- NucleoSpin Plasmid DNA, RNA, and protein purification Kit
- NucleoSpin Gel and PCR Clean-up Kit
- YPD medium, SD-min medium and minimal medium with galactosidase
- NaOH agarose gel and buffer
- Synthetic alpha-factor
- CuSO4
Used methods
- Transformation
- Miniprep
- Restriction digest
- Ligation
- NucleoSpin Gel Clean-up
- NucleoSpin Plasmid DNA purification
- Flow cytometry
All used protocols can be found at the Protocols page.
Used software
- CFlow Plus
- Microsoft Excel
- MATLAB®
Construction
Receptors
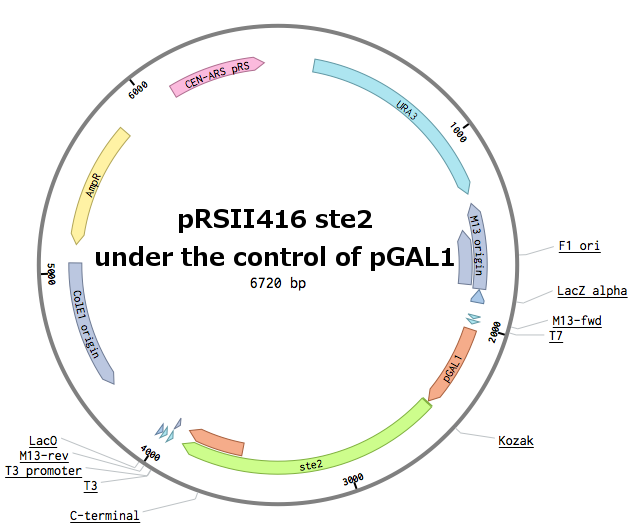
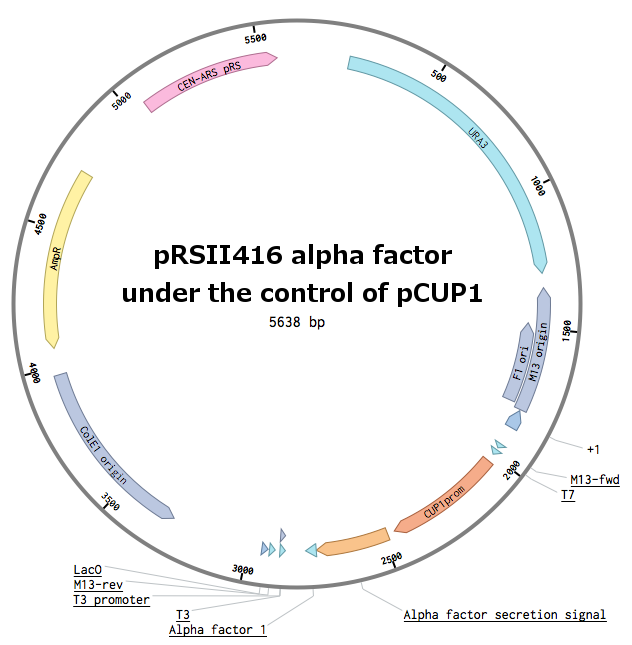
Receptors were constructed by a restriction/ligation using pRSII416 vector. Wild-type Ste2 from Saccharomyces cerevisiae was obtained by a PCR from the genome (the sequence can be found as [http://www.yeastgenome.org/locus/S000001868/overview YFL026W]), pGAL1 promoter was obtained from the Registry (part [http://parts.igem.org/Part:BBa_J63006 BBa_J63006]) and amplified with primers with different restriction sites. Other STE2 receptors were ordered as gBlocks.
First, the plasmid pGAL1_SC_WT was constructed (details can be found in our Notebook):
Then, the Ste2 gene from Saccharomyces cerevisiae was replaced by the different Ste2 sequences. After this cloning, we had plasmids with 6 different Ste2 receptors under the pGAL1 promoter.
In parallel, pGAL1 promoters were replaced with constitutive pADH1 promoters on all plasmids in order to simplify future experiments. The receptor sequences and their promoters were further transferred into pRSII415 plasmids (with LEU marker instead of URA). This made up for 18 different receptor plasmids in total.
Used primers
| Name | Sequence |
|---|---|
| ig2-ste2p-HindIII-F | tcaatgaagcttTACAAAATGTCTGATGCGGCTCCT |
| ig2-ste2p-BamHI-R | tcagatggatccTCATAAATTATTATTATCTTCAGTCCAGAACT |
| ig2-pGAL1-XhoI-F | tacagcctcgagCGGATTAGAAGCCGCCGAG |
| ig2-pGAL1-HindIII-R | tcacgtaagcttCTCCTTGACGTTAAAGTATAGAGGT |
Used sequences
pGAL1 amplified from [http://parts.igem.org/Part:BBa_J63006 BBa_J63006]
tacagcctcgaCGGATTAGAAGCCGCCGAGCGGGTGACAGCCCTCCGAAGGAAGACTCTCCTCCGTGCGTCCTCGTCTTCACCGGTCGCGTTCCTGAAACGCAGATGTGCCTCGCGCCGCACTGCTCCGAACAATAAAGATTCTACAATACTAGCTTTTATGGTTATGAAGAGGAAAAATTGGCAGTAACCTGGCCCCACAAACCTTCAAATGAACGAATCAAATTAACAACCATAGGATGATAATGCGATTAGTTTTTTAGCCTTATTTCTGGGGTAATTAATCAGCGAAGCGATGATTTTTGATCTATTAACAGATATATAAATGCAAAAACTGCATAACCACTTTAACTAATACTTTCAACATTTTCGGTTTGTATTACTTCTTATTCAAATGTAATAAAAGTATCAACAAAAAATTGTTAATATACCTCTATACTTTAACGTCAAGGAGaagcttacgtga
pADH1 amplified from the genome
ctcgagCAACTTCTTTTCTTTTTTTTTCTTTTCTCTCTCCCCCGTTGTTGTCTCACCATATCCGCAATGACAAAAAAATGATGGAAGACACTAAAGGAAAAAATTAACGACAAAGACAGCACCAACAGATGTCGTTGTTCCAGAGCTGATGAGGGGTATCTCGAAGCACACGAAACTTTTTCCTTCCTTCATTCACGCACACTACTCTCTAATGAGCAACGGTATACGGCCTTCCTTCCAGTTACTTGAATTTGAAATAAAAAAAAGTTTGCTGTCTTGCTATCAAGTATAAATAGACCTGCAATTATTAATCTTTTGTTTCCTCGTCATTGTTCTCGTTCCCTTTCTTCCTTGTTTCTTTTTCTGCACAATATTTCAAGCTATACCAAGCATACAATCAACTaagctt
Saccharomyces cerevisiae (SC) STE2 with its (SC) C-terminus
tcaatgaagcttTACAAAATGTCTGATGCGGCTCCTTCATTGAGCAATCTATTTTATGATCCAACGTATAATCCTGGTCAAAGCACCATTAACTACACTTCCATATATGGGAATGGATCTACCATCACTTTCGATGAGTTGCAAGGTTTAGTTAACAGTACTGTTACTCAGGCCATTATGTTTGGTGTCAGATGTGGTGCAGCTGCTTTGACTTTGATTGTCATGTGGATGACATCGAGAAGCAGAAAAACGCCGATTTTCATTATCAACCAAGTTTCATTGTTTTTAATCATTTTGCATTCTGCACTCTATTTTAAATATTTACTGTCTAATTACTCTTCAGTGACTTACGCTCTCACCGGATTTCCTCAGTTCATCAGTAGAGGTGACGTTCATGTTTATGGTGCTACAAATATAATTCAAGTCCTTCTTGTGGCTTCTATTGAGACTTCACTGGTGTTTCAGATAAAAGTTATTTTCACAGGCGACAACTTCAAAAGGATAGGTTTGATGCTGACGTCGATATCTTTCACTTTAGGGATTGCTACAGTTACCATGTATTTTGTAAGCGCTGTTAAAGGTATGATTGTGACTTATAATGATGTTAGTGCCACCCAAGATAAATACTTCAATGCATCCACAATTTTACTTGCATCCTCAATAAACTTTATGTCATTTGTCCTGGTAGTTAAATTGATTTTAGCTATTAGATCAAGAAGATTCCTTGGTCTCAAGCAGTTCGATAGTTTCCATATTTTACTCATAATGTCATGTCAATCTTTGTTGGTTCCATCGATAATATTCATCCTCGCATACAGTTTGAAACCAAACCAGGGAACAGATGTCTTGACTACTGTTGCAACATTACTTGCTGTATTGTCTTTACCATTATCATCAATGTGGGCCACGGCTGCTAATAATGCATCCAAAACAAACACAATTACTTCAGACTTTACAACATCCACAGATAGGTTTTATCCAGGCACGCTGTCTAGCTTTCAAACTGATAGTATCAACAACGATGCTAAAAGCAGTCTCAGAAGTAGATTATATGACCTATATCCTAGAAGGAAGGAAACAACATCGGATAAACATTCGGAAAGAACTTTTGTTTCTGAGACTGCAGATGATATAGAGAAAAATCAGTTTTATCAGTTGCCCACACCTACGAGTTCAAAAAATACTAGGATAGGACCGTTTGCTGATGCAAGTTACAAAGAGGGAGAAGTTGAACCCGTCGACATGTACACTCCCGATACGGCAGCTGATGAGGAAGCCAGAAAGTTCTGGACTGAAGATAATAATAATTTATGAggatccatctga
Candida albicans (CA) STE2 with its (CA) C-terminus (gBlock)
gtaaaacgacggccagtctcgagaagcttTACAAAATGAACATAAATTCTACATTCATTCCAGACAAGCCAGGGGATATCATTATTAGTTATAGTATACCGGGATTGGACCAGCCCATTCAAATACCATTCCATAGTTTAGATTCCTTTCAAACAGACCAAGCTAAGATCGCTTTAGTTATGGGTATCACAATAGGATCTTGTTCTATGACACTTATCTTTCTAATCTCAATCATGTATAAAACGAATAAGTTGACAAACTTGAAGTTGAAACTTAAGTTAAAGTACATCTTGCAATGGATCAACCAAAAGATCTTCACGAAGAAGAGAAATGATAACAAACAGCAACAACAACAGCAACAGCAACAGATTGAGAGTTCTAGTTATAACAATACTACAACGACTACTTCTGGCTCCTATAAACTTTTCCTATTCTATTTAAATTCCCTTATCCTTTTGATTGGGATTATCAGATCAGGTTGTTACCTAAATTACAATTTGGGCCCATTGAATAGCCTGTCATTTGTTTTCACTGGTTGGTATGATGGTAGTAGCTTTATTTCTAGCGATGTTACGAACGGTTTCAAATGCATTCTATATGCCTTGGTAGAAATATCTTTAGGTTTTCAAGTTTACGTAATGTTTAAAACAAGCAACTTGAAAATATGGGGTATAATGGCCAGTTTGTTGAGTATAGGCCTAGGCCTGATCGTGGTCGCGTTTCAAATCAATCTAACGATCTTGTCTCATATAAGGTTCTCTAGGGCTATCTCTACGAATAGATCCGAAGAAGAGAGCTCATTGTCTTCTGATAGCGTGGGTTATGTAATAAATAGCATTTGGATGGATCTGCCAACGATTTTATTTTCCATCAGCATAAATATCATGACAATCTTACTAATTGGTAAGCTAATCATCGCAATTAGAACAAGGAGGTACCTGGGTTTGAAACAGTTCGACTCATTTCACATCTTGTTGATTGGCTTTAGTCAAACGTTAATAATCCCGTCCATCATATTAGTTGTCCACTATTTCTATTTGTCTCAGAACAAGGATAGCTTACTTCAGCAAATTTCCTTATTGTTAATCATATTGATGTTACCGTTATCTTCATTATGGGCACAAACAGCAAACAACACCCATAATATCAACTCTTCTCCCTCACTGTCTTTTATTTCAAGGCATCATTCTTTCGACAGTTCCAGATCCGGAGGTAGTAACACTATTGTGTCCAACGGTGGTAGTAATGGTGGTGGCGGTGGTGGTGGCAACTTCCCAGTAAGTGGTATAGACGCGCAGTTACCGCCTGACATTGAGAAGATCTTACATGAAGATAATAACTATAAACTGCTTAACAGCAATAACGAGTCAGTTAACGACGGCGATATCATTATTAATGACGAGGGGATGATCACTAAGCAGATCACGATTAAAAGAGTTTAAgtcgacggatccactagtggtcatagctgtttcctg
Candida parapsilosis (CP) STE2 with CA C-terminus (gBlock)
gtaaaacgacggccagtctcgagaagcttTACAAAATGAATAAGATAGTCTCAAAACTTTCTTCTAGTGACGTTATAGTCACAGTAACCATTCCAAACGAAGAAGACGGTACTTATGAAGTACCATTCTATGCTATTGATAATTACCATTACAGTCGTATGGAGAACGCAGTGGTGTTGGGTGCCACGATTGGTGCTTGTTCTATGTTGTTAATCATGTTAATCGGGATTCTATTCAAGAATTTTCAAAGGTTAAGGAAATCTCTATTGTTCAACATAAACTTCGCAATTTTATTGATGCTAATTCTGAGATCTGCGTGTTATATTAACTATCTAATGAATAATCTGAGCTCTATATCATTCTTTTTCACCGGTATTTTTGACGATGAGTCATTCATGAGCTCCGATGCCGCAAATGCATTCAAAGTGATCTTAGTAGCATTAATTGAAGTTTCTTTAACCTACCAAATATACGTAATGTTTAAGACCCCCATGTTGAAGTCTTGGGGTATCTTTGCTAGTGTCTTAGCAGGTGTTTTAGGACTTGCAACGTTGGCAACACAAATATATACGACTGTAATGTCTCATGTAAATTTTGTGAACGGCACAACAGGTTCACCTTCACAGGTAACAAGTGCATGGATGGATATGCCAACAATCTTATTTTCTGTTTCTATTAACGTTTTATCTATGTTTTTGGTATGTAAGTTGGGTTTGGCCATTAGAACTAGAAGGTACTTAGGTTTAAAGCAATTCGATGCCTTCCATATTTTATTCATTATGTCAACTCAGACAATGATTATTCCTTCCATAATTTTGTTCGTCCATTACTTCGACCAGAATGATTCACAGACAACCTTGGTTAACATCAGTTTATTGTTAGTGGTCATTAGTTTGCCGTTAAGCTCTTTATGGGCACAAACAGCAAACAACACCCATAATATCAACTCTTCTCCCTCACTGTCTTTTATTTCAAGGCATCATTCTTTCGACAGTTCCAGATCCGGAGGTAGTAACACTATTGTGTCCAACGGTGGTAGTAATGGTGGTGGCGGTGGTGGTGGCAACTTCCCAGTAAGTGGTATAGACGCGCAGTTACCGCCTGACATTGAGAAGATCTTACATGAAGATAATAACTATAAACTGCTTAACAGCAATAACGAGTCAGTTAACGACGGCGATATCATTATTAATGACGAGGGGATGATCACTAAGCAGATCACGATTAAAAGAGTTTAAgtcgacggatccactagtggtcatagctgtttcctg
Candida parapsilosis (CP) STE2 with SC C-terminus (gBlock)
gtaaaacgacggccagtctcgagaagcttTACAAAATGAATAAGATAGTCTCAAAACTTTCTTCTAGTGACGTTATAGTCACAGTAACCATTCCAAACGAAGAAGACGGTACTTATGAAGTACCATTCTATGCTATTGATAATTACCATTACAGTCGTATGGAGAACGCAGTGGTGTTGGGTGCCACGATTGGTGCTTGTTCTATGTTGTTAATCATGTTAATCGGGATTCTATTCAAGAATTTTCAAAGGTTAAGGAAATCTCTATTGTTCAACATAAACTTCGCAATTTTATTGATGCTAATTCTGAGATCTGCGTGTTATATTAACTATCTAATGAATAATCTGAGCTCTATATCATTCTTTTTCACCGGTATTTTTGACGATGAGTCATTCATGAGCTCCGATGCCGCAAATGCATTCAAAGTGATCTTAGTAGCATTAATTGAAGTTTCTTTAACCTACCAAATATACGTAATGTTTAAGACCCCCATGTTGAAGTCTTGGGGTATCTTTGCTAGTGTCTTAGCAGGTGTTTTAGGACTTGCAACGTTGGCAACACAAATATATACGACTGTAATGTCTCATGTAAATTTTGTGAACGGCACAACAGGTTCACCTTCACAGGTAACAAGTGCATGGATGGATATGCCAACAATCTTATTTTCTGTTTCTATTAACGTTTTATCTATGTTTTTGGTATGTAAGTTGGGTTTGGCCATTAGAACTAGAAGGTACTTAGGTTTAAAGCAATTCGATGCCTTCCATATTTTATTCATTATGTCAACTCAGACAATGATTATTCCTTCCATAATTTTGTTCGTCCATTACTTCGACCAGAATGATTCACAGACAACCTTGGTTAACATCAGTTTATTGTTAGTGGTCATTAGTTTGCCGTTAAGCTCTTTATGGGCCACGGCTGCTAATAATGCATCCAAAACAAACACAATTACTTCAGACTTTACAACATCCACAGATAGGTTTTATCCAGGCACGCTGTCTAGCTTTCAAACTGATAGTATCAACAACGATGCTAAAAGCAGTCTCAGAAGTAGATTATATGACCTATATCCTAGAAGGAAGGAAACAACATCGGATAAACATTCGGAAAGAACTTTTGTTTCTGAGACTGCAGATGATATAGAGAAAAATCAGTTTTATCAGTTGCCCACACCTACGAGTTCAAAAAATACTAGGATAGGACCGTTTGCTGATGCAAGTTACAAAGAGGGAGAAGTTGAACCCGTTGACATGTACACTCCCGATACGGCAGCTGATGAGGAAGCCAGAAAGTTCTGGACTGAGGATAATAATAATTTATGAgtcgacggatccactagtggtcatagctgtttcctg
Candida tropicalis (CT) STE2 with CA C-terminus (gBlock)
gtaaaacgacggccagtctcgagaagcttTACAAAATGGACATTAATAACACGATTCAATCTTCCGGCGATATAATCATAACATATACTATTCCAGGTATAGAAGAACCTTTTGAACTACCCTTTGAAGTCTTGAACCATTTTCAATCTGAGCAGTCCAAAAACTGTTTGGTTATGGGAGTTATGATAGGTTCTTGCTCAGTTTTGCTTATCTTCCTGGTAGGGATCTTATTCAAGACTAATAAGTTCTCCACCATAGGTAAGAGTAAGAACTTGTCAAAGAACTTTCTTTTCTATTTGAATTGCTTGATAACATTCATAGGTATTATCAGAGCTGCGTGCTTTTCCAACTATCTTTTAGGCCCATTAAATTCCGCCAGTTTTGCCTTTACAGGCTGGTATAATGGTGAATCTTATGCTAGTTCTGAAGCTGCTAACGGTTTCCGTGTTATCTTGTTCGCATTAATTGAGACTTCAATGGTTTTTCAGGTTTTTGTAATGTTCAGGGGTGCTGGCATGAAAAAACTGGCATATTCCGTGACTATACTGTGTACTGCTTTGGCTTTAGTGGTTGTGGGTTTCCAAATTAATTCAGCTGTTCTTTCTCACAGAAGGTTTGTCAACACCGTTAATGAAATTGGTGACACTGGCTTGTCTTCTATTTGGCTTGATCTACCGACCATACTATTTAGCGTATCTGTCAATTTAATGTCTGTACTTTTGATTGGCAAGTTGATTATGGCAATAAAAACCAGAAGGTATTTGGGTCTAAAACAATTTGACAGCTTCCATGTTCTACTGATTTGCAGCACACAAACCCTTTTGGTGCCTAGTTTGATATTGTTCGTACATTACTTCCTATTTTTTAGAAATGCAAATGTGATGCTGATCAATATTTCCATTTTACTTATTGTTTTGATGCTGCCTTTTTCAAGTCTGTGGGCACAAACAGCAAACAACACCCATAATATCAACTCTTCTCCCTCACTGTCTTTTATTTCAAGGCATCATTCTTTCGACAGTTCCAGATCCGGAGGTAGTAACACTATTGTGTCCAACGGTGGTAGTAATGGTGGTGGCGGTGGTGGTGGCAACTTCCCAGTAAGTGGTATAGACGCGCAGTTACCGCCTGACATTGAGAAGATCTTACATGAAGATAATAACTATAAACTGCTTAACAGCAATAACGAGTCAGTTAACGACGGCGATATCATTATTAATGACGAGGGGATGATCACTAAGCAGATCACGATTAAAAGAGTTTAAgtcgacggatccactagtggtcatagctgtttcctg
Lodderomyces elongisporus (LE) STE2 with SC C-terminus (gBlock)
gtaaaacgacggccagtctcgagaagcttTACAAAATGGATGAAGCAATAAATGCCAATCTGGTCTCCGGGGATATCATTGTTTCATTCAATATACCTGGATTACCAGAGCCTGTTCAAGTGCCATTTTCAGAGTTCGACTCCTTTCATAAGGATCAATTGATTGGAGTTATTATATTAGGCGTCACTATCGGTGCTTGCTCTTTGTTGTTAATACTATTGCTTGGAATGTTGTATAAGTCACGTGAAAAGTATTGGAAGAGCTTATTGTTTATGTTGAATGTTTGTATACTTGCCGCGACAATCTTACGTTCTGGTTGCTTTTTGGATTATTATTTGTCTGATCTAGCTTCTATCAGTTATACCTTTACCGGTGTTTATAACGGCACAAGTTTTGCAAGTAGTGATGCAGCCAATGTTTTCAAGACTATTATGTTTGCACTAATAGAGACGAGTTTGACCTTTCAAGTTTATGTTATGTTCCAAGGCACCACATGGAAGAACTGGGGACATGCAGTAACCGCATTGTCCGGTTTGTTGTCAGTCGCGTCAGTAGCATTCCAGATATATACTACTATATTAAGTCACAATAACTTCAACGCAACAATTTCAGGGACTGGTACTTTAACATCTGGGGTGTGGATGGACCTGCCAACTTTACTATTTGCTGCATCAATCAATTTTATGACTATCCTACTGCTATTCAAACTTGGCATGGCTATCAGACAAAGGAGATACTTAGGTTTGAAACAGTTTGATGGCTTTCACATCCTTTTTATTATGTTTACGCAGACCTTATTTATTCCCTCTATCTTATTGGTGATCCATTATTTTTATCAAGCTATGTCTGGTCCATTCATCATAAACATGGCCCTGTTCCTTGTGGTAGCCTTCTTGCCATTGTCATCATTGTGGGCCACGGCTGCTAATAATGCATCCAAAACAAACACAATTACTTCAGACTTTACAACATCCACAGATAGGTTTTATCCAGGCACGCTGTCTAGCTTTCAAACTGATAGTATCAACAACGATGCTAAAAGCAGTCTCAGAAGTAGATTATATGACCTATATCCTAGAAGGAAGGAAACAACATCGGATAAACATTCGGAAAGAACTTTTGTTTCTGAGACTGCAGATGATATAGAGAAAAATCAGTTTTATCAGTTGCCCACACCTACGAGTTCAAAAAATACTAGGATAGGACCGTTTGCTGATGCAAGTTACAAAGAGGGAGAAGTTGAACCCGTTGACATGTACACTCCCGATACGGCAGCTGATGAGGAAGCCAGAAAGTTCTGGACTGAGGATAATAATAATTTATGAgtcgacggatccactagtggtcatagctgtttcctg
Signals
Plasmids with pheromones were constructed by a restriction/ligation using pRSII416 vector. Wild-type alpha-factor from Saccharomyces cerevisiae was obtained by a PCR from the genome (MF(ALPHA)1 locus - [http://www.yeastgenome.org/locus/mf%28alpha%291/overview YPL187W]).
We could not design the reverse primer for the whole sequence (secretion tag + alpha factor) because of the repeating pheromone sequences. To solve this issue, we firstly amplified the secretion tag only. Using a Band-stab PCR, we added the actual pheromone, stop codon and a restriction site (as a part of the new reverse primer). The pCUP1 promoter was obtained from our laboratory depository.
First, the plasmid pCUP1_SC_alpha was constructed (details can be found in our Notebook):
Subsequently, plasmids with other pheromones were constructed using site-directed mutagenesis.
Used sequences
pCUP1
ctcgagAGATCCCATTACCGACATTTGGGCGCTATACGTGCATATGTTCATGTATGTATCTGTATTTAAAACACTTTTGTATTATTTTTCCTCATATATGTGTATAGGTTTATACGGATGATTTAATTATTACTTCACCACCCTTTATTTCAGGCTGATATCTTAGCCTTGTTACTAGTTAGAAAAAGACATTTTTGCTGTCAGTCACTGTCAAGAGATTCTTTTGCTGGCATTTCTTCTAGAAGCAAAAAGAGCGATGCGTCTTTTCCGCTGAACCGTTCCAGCAAAAAAGACTACCAACGCAATATGGATTGTCAGAATCATATAAAAGAGAAGCAAATAACTCCTTGTCTTGTATCAATTGCATTATAATATCTTCTTGTTAGTGCAATATCATATAGAAGTCATCGAAATAGATATTAAGAAAAACAAACTGTAACGAATTGGGATCCgtcgac
Secretion tag + alpha factor
gtcgacTACAAAATGAGATTTCCTTCAATTTTTACTGCAGTTTTATTCGCAGCATCCTCCGCATTAGCTGCTCCAGTCAACACTACAACAGAAGATGAAACGGCACAAATTCCGGCTGAAGCTGTCATCGGTTACTTAGATTTAGAAGGGGATTTCGATGTTGCTGTTTTGCCATTTTCCAACAGCACAAATAACGGGTTATTGTTTATAAATACTACTATTGCCAGCATTGCTGCTAAAGAAGAAGGGGTATCTTTGGATAAAAGAGAGGCTGAAGCTTGGCATTGGTTGCAACTAAAACCTGGCCAACCAATGTACTAAgaattc
Used primers
| Name | Sequence |
|---|---|
| ig2-AlphaP-CA-F | ggatatttcgaacctggttaagaattcGGAGCTCCAGCTTTTGTTCCC |
| ig2-AlphaP-CA-R | aaaatttgttaaacggaagccAGCTTCAGCCTCTCTTTTATCCAAAG |
| ig2-AlphaP-CP-F | ggttactatgaaccacagtaagaattcGGAGCTCCAGCTTTTGTTCCC |
| ig2-AlphaP-CP-R | gtaggttgtccaatgaggcttAGCTTCAGCCTCTCTTTTATCCAAAG |
| ig2-AlphaP-CT-F | atatggatggttttctccaaactaagaattcGGAGCTCCAGCTTTTGTTCCC |
| ig2-AlphaP-CT-R | ctggttaatctgaatttgaatttggcAGCTTCAGCCTCTCTTTTATCCAAAG |
| ig2-AlphaP-LE-F | ggaaggtttagtcctgtttaagaattcGGAGCTCCAGCTTTTGTTCCC |
| ig2-AlphaP-LE-R | atatcttgtccacatccaaccAGCTTCAGCCTCTCTTTTATCCAAAG |
Pheromones
| Name | Sequence |
|---|---|
| Saccharomyces cerevisiae | TGGCATTGGTTGCAACTAAAACCTGGCCAACCAATGTAC |
| Candida albicans | GGCTTCCGTTTAACAAATTTTGGATATTTCGAACCTGGT |
| Candida parapsilosis | AAGCCTCATTGGACAACCTACGGTTACTATGAACCACAG |
| Candida tropicalis | GCCAAATTCAAATTCAGATTAACCAGATATGGATGGTTTTCTCCAAAC |
| Lodderomyces elongisporus | GGTTGGATGTGGACAAGATATGGAAGGTTTAGTCCTGTT |
Validation
During the construction, all intermediate constructs and final plasmids were immediately verified by a restriction digest and/or sequencing (details can be accessed in our Notebook).
Function was validated by the following experiments.
Receptors
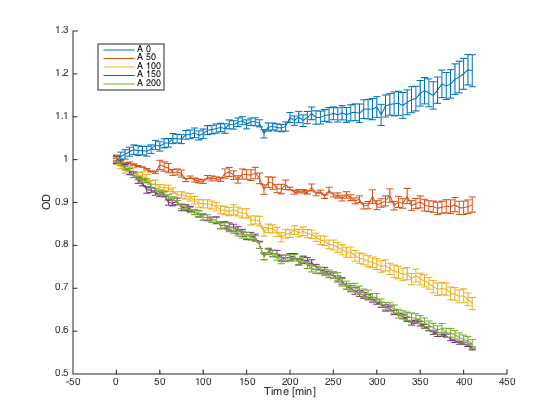
Wild-type MATa cells (7284) and alpha cells with pRSII416_pGAL1_STE2 were grown in minimal media with galactosidase overnight. At time t=0, cultures were diluted and different concentrations of the synthetic alpha pheromone were added (0, 50, 100, 150 and 200 μM). In the ideal case, alpha cells with introduced STE2 receptor should exhibit similar behavior as WT MATa cells - growth arrest in the presence of the pheromone.
Reaction of the WT MATa cells to different concentrations of the pheromone:
Reaction of the alpha cells with introduced STE2 receptor to different concentrations of the pheromone:
As can be seen, the alpha cells producing STE2 receptor for the alpha factor showed a similar behavior to MATa cells that possess this receptor naturally.
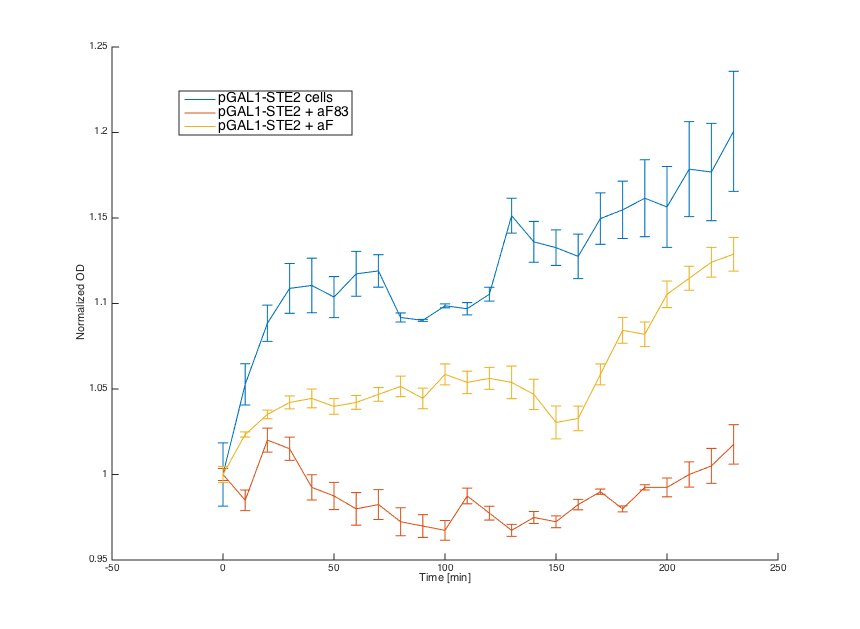
Signals
Wild-type MATa cells (7284) , cells with pRSII416_pGAL1_STE2, cells with pCUP1_SC_Alpha and wild-type alpha cells (7283) were grown overnight in SD min.
In the morning, cultures 7283, 7284 and pRSII416_pGAL1_STE2 were diluted in the same medium and incubated for 6 hours. Culture pCUP1_SC_Alpha was centrifuged (3000 g) and resuspended in SG-min (minimal medium with galactose) supplemented with 0.1 μM CuSO4.
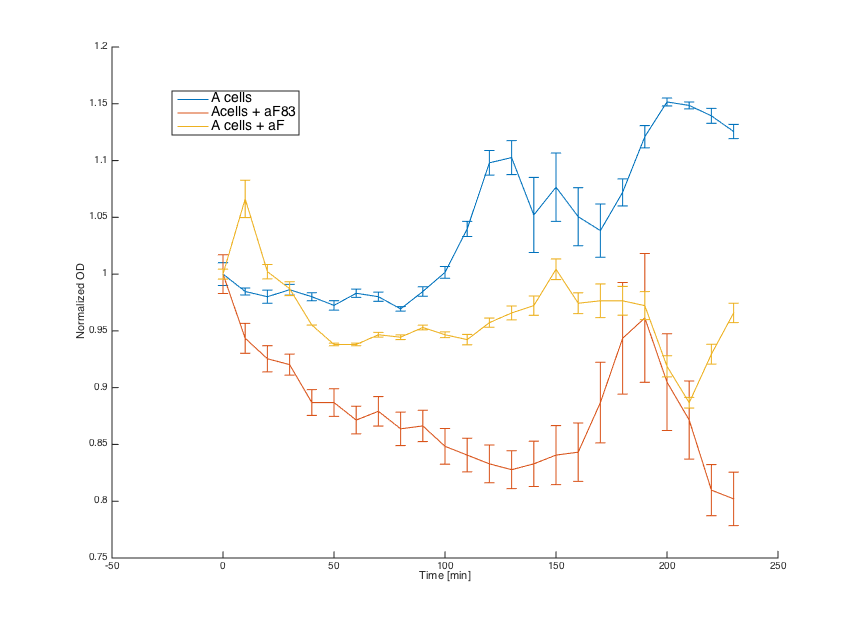
Alpha factors for induction were obtained by centrifugation of 7283 (aF83) and pCUP1_SC_Alpha (aF) cultures at 3000 g and by removing the cell pellet. Obtained supernatant was combined with MATa and pRSII416_pGAL1_STE2 cells (20μL culture + 80μL media).
Firstly, reaction of the MATa cells in the medium with WT alpha factor and the alpha factor produced by our MATa cells transformed with pCUP1_SC_Alpha was determined:
The MATa cells without any pheromone present exhibited normal growth after 1 hour from the transfer to the new medium. Cells with a WT pheromone present were arrested in their growth as expected. The same reaction was observed for cells in the medium with pheromones produced by the MATa cells with our pCUP1_SC_Alpha plasmid, even though on a smaller scale. This could be explained by a lower production of the pheromone from the partially induced pCUP1 promoter.
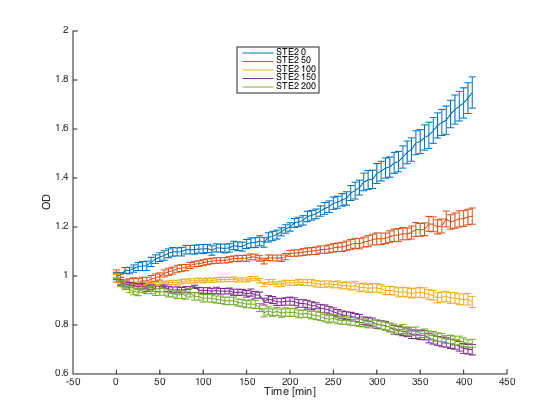
Then we tested reaction of the alpha cells transformed with the pRSII416_pGAL1_STE2 plasmid in both media:
Cells in media without any pheromone exhibited normal growth after 1 hour from the transfer to the new medium. Cells in media with WT pheromone exhibited growth arrest as expected. Cells in media with pheromones produced by MATa cells thanks to our pCUP1_SC_Alpha plasmid showed only a partial arrest, which corresponds to the lower production of the pheromone (as mentioned above).
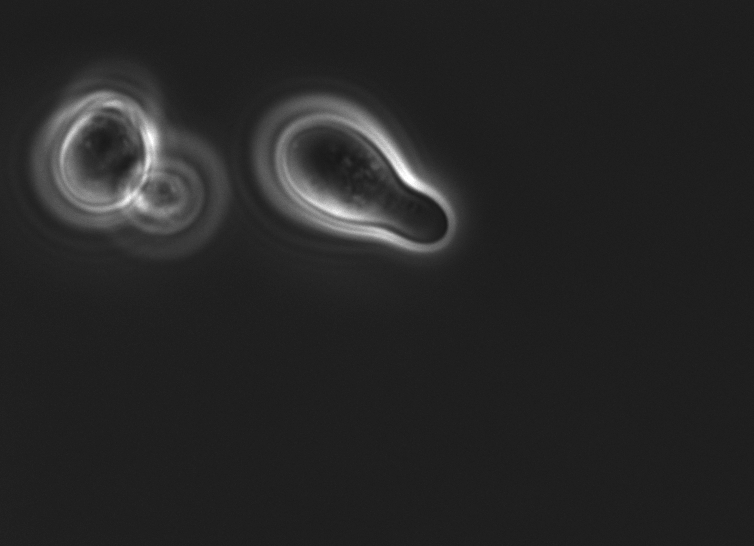
Also, MATa cells transformed with the pCUP1_SC_Alpha were examined under the microscope:
Summary: Coupling of the introduced STE2 receptor to the pheromone mating pathway, and successful production and secretion of the introduced mating pheromone to MATa cells were demonstrated.
Results
Test of orthogonality (growth arrest approach)
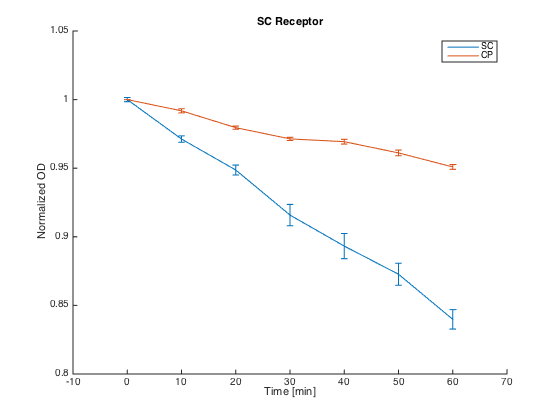
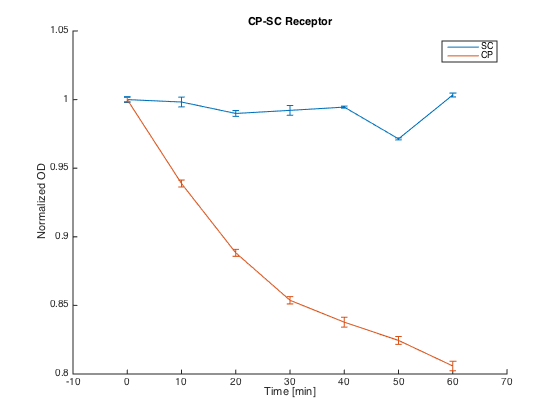
To test the orthogonality of the two most promising receptors (SC and CP-SC), we conducted an assay using growth arrest as the indicator of an STE2 receptor activation.
Both receptors in MATa cells (7284) and the corresponding pheromones in MATa cells (Δbar1) were incubated for 60 h in SD-min media, supplemented with proper amino acids.
After incubation, cultures with pheromones were centrifuged (3000 g) and transferred into SG-min media supplemented with AAs and 100 μM CuSO4 in order to induce the pheromone production. After 4 h, their OD was measured and cultures were centrifuged (4000 g). The supernatants with produced pheromones were diluted to the same concentration (according to the OD measured) and kept at RT during the plate preparation.
At the same time, cultures with receptors were centrifuged (3000 g) and transferred into SG-min media (300 μL). Each well thus contained 20 μL of the receptor culture and 80 μL of the media with a single pheromone.
Measurements were done every ten minutes, shaking was set to medium and the temperature was 30°C.
The measured responses to both pheromones are shown here:
As can be seen, both receptors were activated only by their corresponding pheromones. This demonstrates the orthogonality of these two receptors.
Summary: We have experimentally verified the orthogonality of the two of our receptors.
Test of orthogonality (fluorescence approach)
Since the orthogonality tests using the growth arrest allowed us to verify orthogonality of only two receptors, we decided to continue these assays using fluorescence as the output.
We transformed MATa cells (Δbar1) with the pRSII415_pADH1_Receptor plasmids (to produce and display different receptors on the cell) together with the pFUS1_GFP plasmid (to detect the activation of MAPK by an increase of fluorescence).
| SC alpha | CA alpha | CP alpha | CT alpha | LE alpha | |
|---|---|---|---|---|---|
| SC R | 45622 | 28037 | 26164 | 29616 | 26978 |
| CA R | 28468 | 128690 | 39478 | 14365 | 49222 |
| CP R | 33231 | 21858 | 61210 | 18784 | 13884 |
| CT R | 17141 | 19921 | 19769 | 54829 | 16639 |
| LE R | 6849 | 13800 | 12884 | 11659 | 45527 |
Final constructs
Our module has developed many plasmids throughout the summer. The main contribution is the creation of a library of different STE2 receptors and their corresponding pheromones that can be functionally expressed in Saccharomyces cerevisiae. All plasmids were verified using restriction digest, gel electrophoresis, and DNA sequencing.
To recapitulate, we present here a complete list of plasmids.
Receptors
- Saccharomyces cerevisiae with its natural C-terminus
- Candida albicans with its natural C-terminus
- Candida parapsilosis with C-terminus from Candida albicans
- Candida parapsilosis with C-terminus from Saccharomyces cerevisiae
- Candida tropicalis with C-terminus from Candida albicans
- Lodderomyces elongisporus with C-terminus from Saccharomyces cerevisiae
All the receptors above were put under the control of the inducible pGAL1 and constitutive pADH1 promoter. Receptors under the control of pADH1 were also put into the pRSII415 vector (to change the selectable marker from URA to LEU).
In total, 18 plasmids were constructed.
Pheromones
Plasmids with a secretion tag and pheromones from these species were constructed:
- Saccharomyces cerevisiae
- Candida albicans
- Candida parapsilosis
- Candida parapsilosis
- Candida tropicalis
- Lodderomyces elongisporus
All pheromones were put under the control of the inducible pCUP1 promoter and pGAL1 promoter. Pheromones with pCUP1 promoter were also put into the pRSII415 vector (to change the selectable marker from URA to LEU).
In total, 15 plasmids were constructed.
References
- ↑ Lin, C.-H., Choi, a., & Bennett, R. J. (2011). Defining pheromone-receptor signaling in Candida albicans and related asexual Candida species. Molecular Biology of the Cell, 22(24), 4918–4930. doi:10.1091/mbc.E11-09-0749
- ↑ Caplan, S., Green, R., Rocco, J., & Kurjan, J. (1991). Glycosylation and structure of the yeast MF alpha 1 alpha-factor precursor is important for efficient transport through the secretory pathway. Journal of Bacteriology, 173, 627–635. doi:10.1039/c1mb05175j
- ↑ Kurjan J, Herskowitz I. (1982) Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933-43. doi:10.1016/0092-8674(82)90298-7
- ↑ Caplan, S., Green, R., Rocco, J., & Kurjan, J. (1991). Glycosylation and structure of the yeast MF alpha 1 alpha-factor precursor is important for efficient transport through the secretory pathway. Journal of Bacteriology, 173, 627–635. doi:10.1039/c1mb05175j
- ↑ Janiak, A. M., Sargsyan, H., Russo, J., Naider, F., Hauser, M., & Becker, J. M. (2005). Functional expression of the Candida albicans alpha-factor receptor in Saccharomyces cerevisiae. Fungal Genetics and Biology, 42(2005), 328–338. doi:10.1016/j.fgb.2005.01.006