Team:Czech Republic/Project/Orthogonal signals and receptors
Synthetic signals and receptors
Intro / Background
Test [Janiak2005]. [Lin2011]
Design
Concept
Receptors
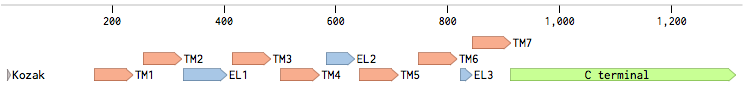
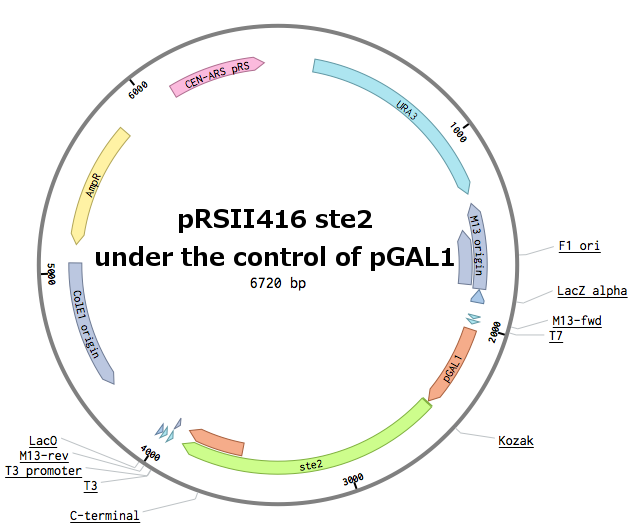
Alpha factor receptor (STE2) is produced and displayed by a cells. It is a 431aa long seven transmembrane-domain (TM in the figure) GPCR. Since the sequence is known, it can be easily obtained by a PCR from the genome of our Saccharomyces cerevisiae strain. For higher translation in the yeast, we added consensus Kozak sequence before the start codon. Also, restriction sites were added to the ends of the fragment to allow easy future cloning.
Signals
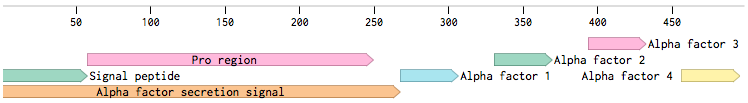
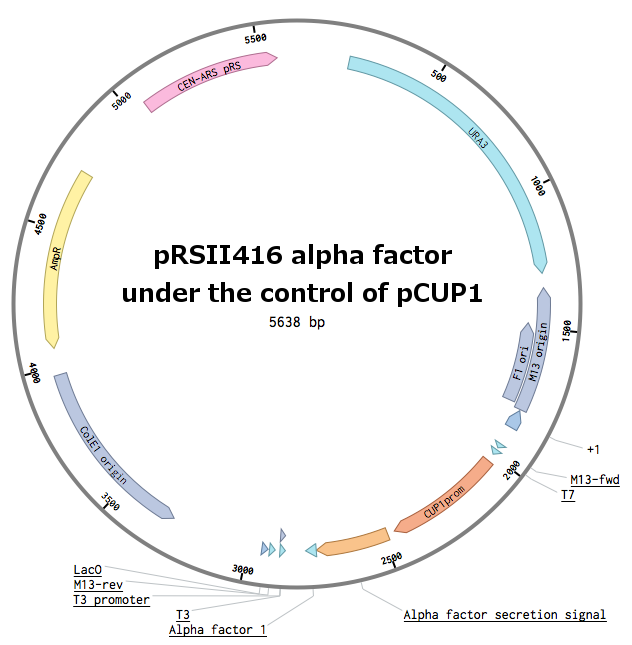
Alpha-factor mating pheromone is a short peptide (typically 13aa long) secreted by alpha cells. For our project, we needed to produce a variety of these pheromones and secrete them from the cells. Altough there are some secretion tags present in the registry (BBa_K416003, BBa_K792002), there are no parts encoding pheromones available. Also, since we found evidence that the complete wild-type signal tag is needed for the proper secretion of the pheromone [Caplan1991], we decided to obtain the whole sequence directly from the MF(ALPHA)1 locus.
MF(ALPHA)1 gene codes for four mature alpha-factors within a putative precursor of 165 amino acids. This sequence begins with a signal tag for secretion and a segment with three glycosylation sites (89aa). The second segment contains four mature alpha-factors, each preceded by spacer peptides, which are contain proteolytic processing signals [Kurjan1982].
Since we wanted the pheromone peptides to be easily changeable by Site-directed mutagenesis (SDM), we decided to preserve the signal tag with only one copy of the pheromone:
This would create a unique site for the SDM. The final production level of the pheromone is supposed to be practically the same, since the used plasmid is present in the cell in more copies (2-5). Also, for higher expression in the cells, we added consensus Kozak sequence before the start codon. Restriciton sites were added to the ends of the sequence for easy future cloning.
DNA
Receptors
Genomic PCR of WT SC-STE2 ([http://www.yeastgenome.org/locus/S000001868/overview YFL026W]) - ORF
Signals
Genomic PCR of MF(ALPHA)1 ([http://www.yeastgenome.org/locus/mf%28alpha%291/overview YPL187W]) - secretion tag (first PCR), second PCR to add the actual pheromone and stop codon
Materials and methods
Used strains
Used material
Used methods
Used software
Construction
Validation
Receptors
Signals
Wild-type a cells (7284) , cells with pGAL1_STE2, cells with pCUP1_Pheromone and wild-type alpha cells (7283) were grown overnight in SD min.
In the morning, cultures 7283, 7284 and pGAL1_STE2 were diluted in the same medium and incubated for 6 hours. Culture pCUP1_Pheromone was centrifuged (3000g) and resuspended in SG-min (minimal medium with galactose) supplemented with 0.1μM CuSO4.
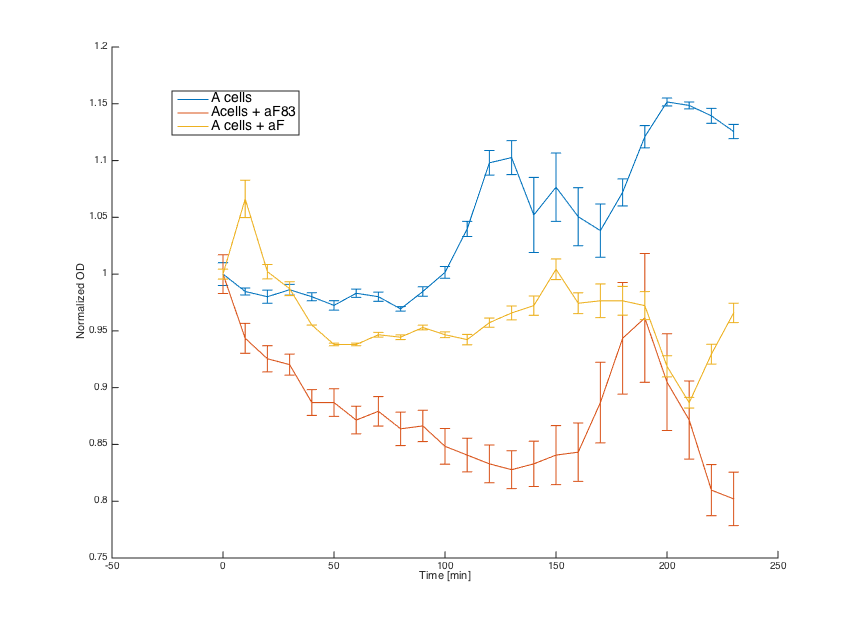
Alpha factors for induction were obtained by centrifugation of 7283 (aF83) and pCUP1_Pheromone (aF) cultures at 3000g and removing the cell pellet. Obtained supernatant was combined with a and pGAL1_STE2 cells (20μL culture + 80μL media).
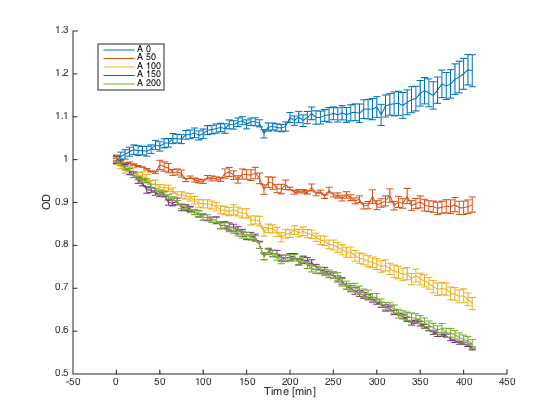
First, reaction of the a cells in the medium with WT alpha factor and the alpha factor produced by our a cells transformed with pCUP1_Pheromone was determined:
The a cells without any pheromone present exhibited normal growth after 1h from the transfer to the new medium. Cells with a WT pheromone present were arrested in their grow as expected. The same reaction was observed for the cells in the medium with pheromones produced by the a cells with our pCUP1_Pheromone plasmid, even in a smaller scale. This could be explained by a smaller production of the pheromone from the partially induced pCUP1 promoter.
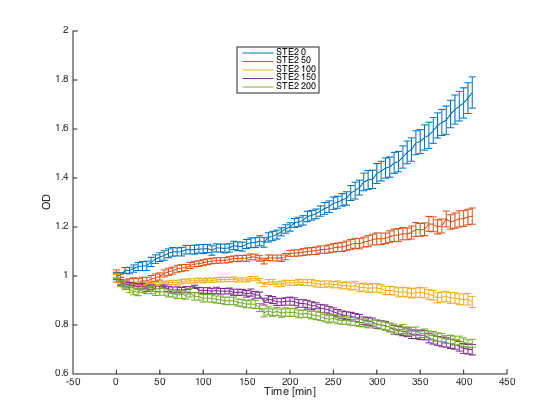
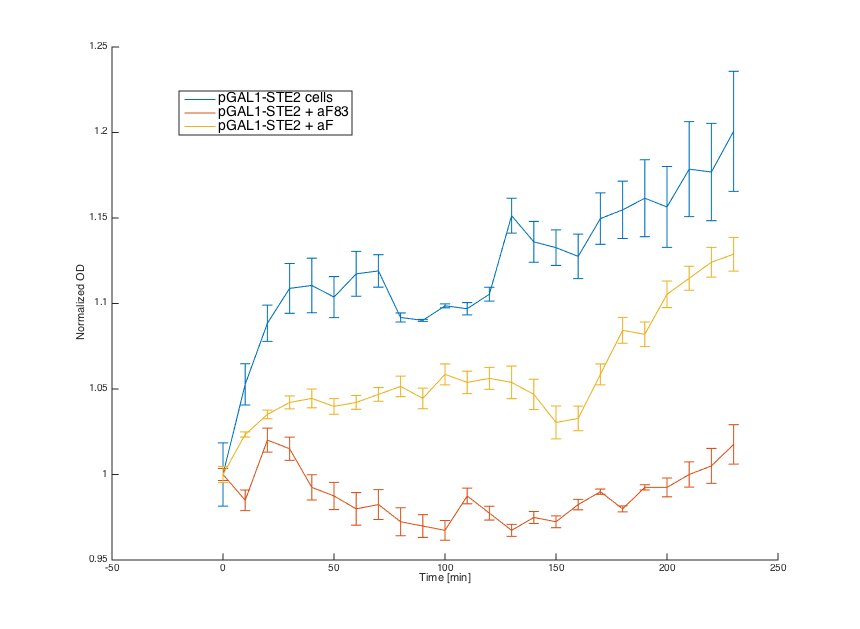
Then, we tested reaction of the alpha cells transformed with pGAL1_STE2 plasmid in both media:
Cells in media without any pheromone exhibited normal growth after 1h from the transfer to the new medium. Cells in the media with WT pheromone exhibited growth arrest as expected and the cellls in media with pheromones produced by a cells with our pCUP1_Pheromone plasmid exhibited only partial arrest, which corresponds to the lower production of the pheromone (as mentioned above).
Results
Test of orthogonality (growth arrest approach)
Test of orthogonality (fluorescence approach)
Final constructs
References
- ↑ Lin, C.-H., Choi, a., & Bennett, R. J. (2011). Defining pheromone-receptor signaling in Candida albicans and related asexual Candida species. Molecular Biology of the Cell, 22(24), 4918–4930. doi:10.1091/mbc.E11-09-0749
- ↑ Caplan, S., Green, R., Rocco, J., & Kurjan, J. (1991). Glycosylation and structure of the yeast MF alpha 1 alpha-factor precursor is important for efficient transport through the secretory pathway. Journal of Bacteriology, 173, 627–635. doi:10.1039/c1mb05175j
- ↑ Kurjan J, Herskowitz I. (1982) Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933-43. doi:10.1016/0092-8674(82)90298-7
- ↑ Caplan, S., Green, R., Rocco, J., & Kurjan, J. (1991). Glycosylation and structure of the yeast MF alpha 1 alpha-factor precursor is important for efficient transport through the secretory pathway. Journal of Bacteriology, 173, 627–635. doi:10.1039/c1mb05175j
- ↑ Janiak, A. M., Sargsyan, H., Russo, J., Naider, F., Hauser, M., & Becker, J. M. (2005). Functional expression of the Candida albicans alpha-factor receptor in Saccharomyces cerevisiae. Fungal Genetics and Biology, 42(2005), 328–338. doi:10.1016/j.fgb.2005.01.006