Difference between revisions of "Team:Exeter/Results"
| (13 intermediate revisions by the same user not shown) | |||
| Line 86: | Line 86: | ||
captions: true, | captions: true, | ||
adaptiveHeight: true, | adaptiveHeight: true, | ||
| − | preloadImages: 'all' | + | preloadImages: 'all', |
| + | pager: 'false' | ||
}); | }); | ||
| Line 96: | Line 97: | ||
captions: true, | captions: true, | ||
adaptiveHeight: true, | adaptiveHeight: true, | ||
| − | preloadImages: 'all' | + | preloadImages: 'all', |
| + | pager: 'false' | ||
}); | }); | ||
| Line 106: | Line 108: | ||
captions: true, | captions: true, | ||
adaptiveHeight: true, | adaptiveHeight: true, | ||
| − | preloadImages: 'all' | + | preloadImages: 'all', |
| + | pager: 'false' | ||
}); | }); | ||
| Line 168: | Line 171: | ||
<h4><em>In silico</em> testing:</h4> | <h4><em>In silico</em> testing:</h4> | ||
| + | |||
| + | |||
| + | To see an explanation of <em>in silico</em> testing, please visit the <a href="https://2015.igem.org/Team:Exeter/Modeling">modelling page</a>. | ||
| + | |||
| + | Download raw data files for Zeus NUPACK testing <a href="https://static.igem.org/mediawiki/2015/c/c3/Exeter_ZEUSJ_NuPACK.zip">here</a>. | ||
| + | Visit the <a href="http://parts.igem.org/Part:BBa_K1586003">registry page</a> for more information. | ||
</div> | </div> | ||
| Line 174: | Line 183: | ||
<div id="validation"> | <div id="validation"> | ||
| + | |||
| + | |||
| + | |||
| + | <h4>Experimental validation of GreenFET1J (<a href="https://2015.igem.org/Team:Exeter/Parts#toeholds">BBa_K1586000</a>):</h4> | ||
| + | |||
<div class="containerSlider300" style="float:right"> | <div class="containerSlider300" style="float:right"> | ||
| Line 182: | Line 196: | ||
</div> | </div> | ||
</div> | </div> | ||
| − | |||
| − | |||
<div class="containerSlider400" style="float:left"> | <div class="containerSlider400" style="float:left"> | ||
| Line 196: | Line 208: | ||
<p> | <p> | ||
| − | As was explained on the <a href="">experiments page</a>, GreenJ's (BBa_K1586000) function was characterised and validated by measuring fluorescence intensity of GFP in response to increasing amounts of trigger RNA. Shown in figure 1 is the raw data which was collected from this experiment. In order to analyse this data, the log10 of the amounts of TrigGreen (in nanograms) were calculated. So that the log10 value for 0ng could be calculated, | + | As was explained on the <a href="">experiments page</a>, GreenJ's (BBa_K1586000) function was characterised and validated by measuring fluorescence intensity of GFP in response to increasing amounts of trigger RNA. Shown in figure 1 is the raw data which was collected from this experiment. In order to analyse this data, the log10 of the amounts of TrigGreen (in nanograms) were calculated. So that the log10 value for 0ng could be calculated, 1x10<sup>-6</sup>ng was used instead, and all values were increased by six so that 0ng shows as 0 on the graph. These data were then plotted on a graph and a linear trend-line fitted (figure 2). |
<div class="containerSlider300" style="float:left"> | <div class="containerSlider300" style="float:left"> | ||
<div class="sliderstatic300"> | <div class="sliderstatic300"> | ||
| − | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/ | + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/7/7e/Exeter_percentage_greenJ.png"><img src="https://static.igem.org/mediawiki/2015/7/7e/Exeter_percentage_greenJ.png" title="Figure 3: Graph of log<sub>10</sub>(TrigGreen)+6 vs. fluorescence intensity, including percentage increase."></a></div> |
</div> | </div> | ||
</div> | </div> | ||
| − | On this graph, the point at about 7.72 (52.31 ng) does not match the general trend of the other points, in fact it registers at about the same fluorescence intensity as 0ng of trigger RNA. Due to the low volumes of RNA which were being added to the reactions, this reading could easily be due to a pipetting error and has therefore been left out of any further analysis. A graph without this value and with a newly fitted trend-line is shown in figure 3 | + | On this graph, the point at about 7.72 (52.31 ng) does not match the general trend of the other points, in fact it registers at about the same fluorescence intensity as 0ng of trigger RNA. Due to the low volumes of RNA which were being added to the reactions, this reading could easily be due to a pipetting error and has therefore been left out of any further analysis. A graph without this value and with a newly fitted trend-line is shown in figure 3, as can been seen on the graph, the R<sup>2</sup> value for this is 0.7247. Also shown is the percentage increase in fluorescence between no trigger and the 2615.23 ng of trigger; 138%.</br> |
</br> | </br> | ||
From this graph, it can be seen that the as the amount of TrigGreen increases, so does GFP fluorescence intensity, hence validating that <b>BBa_K1586000 works as expected</b>. | From this graph, it can be seen that the as the amount of TrigGreen increases, so does GFP fluorescence intensity, hence validating that <b>BBa_K1586000 works as expected</b>. | ||
| + | </br> | ||
| + | </br> | ||
</div> | </div> | ||
| Line 215: | Line 229: | ||
<div id="further_characterisation"> | <div id="further_characterisation"> | ||
| + | <div style="float:right"> | ||
| + | <img src="https://static.igem.org/mediawiki/2015/b/bb/Exeter_chromoplates.png" width="200"> | ||
| + | |||
| + | </div> | ||
<h2>Further characterisation:</h2> | <h2>Further characterisation:</h2> | ||
| + | |||
| + | |||
| + | <p> | ||
| + | The following parts were further characterised: | ||
| + | <ul> | ||
| + | <li><a href="http://parts.igem.org/Part:BBa_K1073020">aeBlue</a></li> | ||
| + | <li><a href="http://parts.igem.org/Part:BBa_K1073022">eforRed</a></li> | ||
| + | <li><a href="http://parts.igem.org/Part:BBa_K1431814">amajLime</a></li> | ||
| + | </ul> | ||
| + | </p> | ||
<h4>Absorbance spectra:</h4> | <h4>Absorbance spectra:</h4> | ||
| + | |||
| + | |||
| + | <div class="containerSlider300" style="float:left"> | ||
| + | <div class="slider300"> | ||
| + | |||
| + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/4/4b/Exeter_aeBlue_spectrum.png"><img src="https://static.igem.org/mediawiki/2015/4/4b/Exeter_aeBlue_spectrum.png" title="Figure 4: Spectrum for aeBlue, peak maxima at 595nm."></a></div> | ||
| + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/4/40/EforRed_spectrum.png"><img src="https://static.igem.org/mediawiki/2015/4/40/EforRed_spectrum.png" title="Figure 4: Spectrum for eforRed, peak maxima at 580nm."></a></div> | ||
| + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/4/4b/Exeter_aeBlue_spectrum.png"><img src="https://static.igem.org/mediawiki/2015/4/4b/Exeter_aeBlue_spectrum.png" title="Figure 4: Spectrum for amajLime, peak maxima at 595nm."></a></div> | ||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | <p> | ||
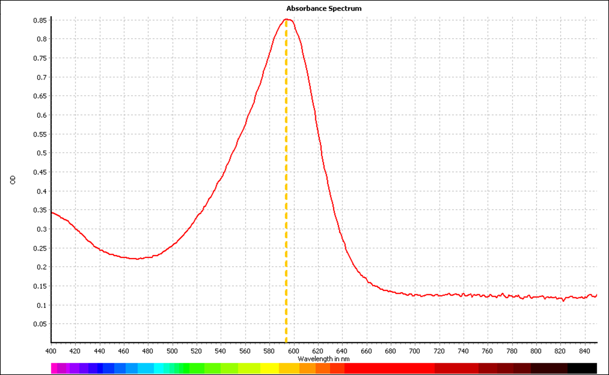
| + | Shown in figure 4 are the absorbance spectra for aeBlue, eforRed, and amajLime between 400nm and 850nm. The peak maxima for these are 595nm, 580nm, and 595nm respectively. | ||
| + | </p> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| Line 227: | Line 277: | ||
| + | <div class="containerSlider300" style="float:left"> | ||
| + | <div class="slider300"> | ||
| + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/8/86/Exeter_amajLime_standard_curve.png"><img src="https://static.igem.org/mediawiki/2015/8/86/Exeter_amajLime_standard_curve.png" title="Figure 5: Standard curve for amajLime measured at 458nm."></a></div> | ||
| + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/a/a0/Exeter_aeBlue_standard_curve.png"><img src="https://static.igem.org/mediawiki/2015/a/a0/Exeter_aeBlue_standard_curve.png" title="Figure 5: Standard curve for aeBlue measured at 595nm"></a></div> | ||
| + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/d/dc/Exeter_eforRed_standard_curve.png"><img src="https://static.igem.org/mediawiki/2015/d/dc/Exeter_eforRed_standard_curve.png" title="Figure 5: Standard curve for eforRed measured at "></a></div> | ||
| + | </div> | ||
</div> | </div> | ||
| + | <p> | ||
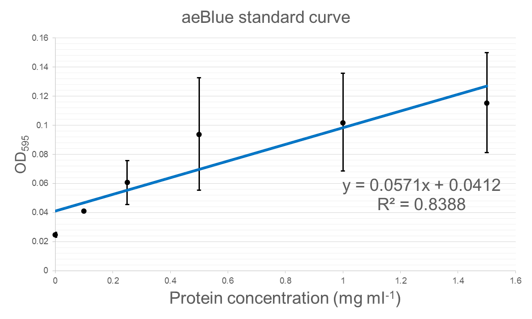
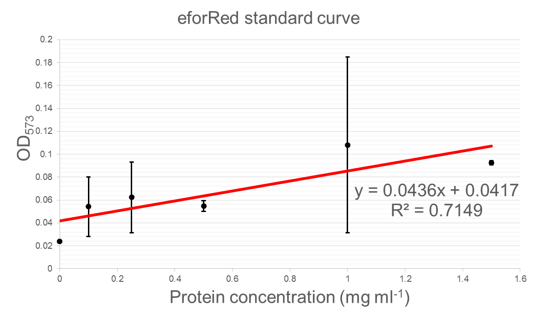
| + | Figure 5 shows standard curves of concentration in mg ml<sup>-1</sup> vs. OD at peak maxima for each of the three chromoproteins further characterised. | ||
| + | </p> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | <h4>Visual limits:</h4> | ||
| + | |||
| + | <div class="containerSlider300" style="float:left"> | ||
| + | <div class="slider300"> | ||
| + | |||
| + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/4/4a/Exeter_amajLime_visual_limit.png"><img src="https://static.igem.org/mediawiki/2015/4/4a/Exeter_amajLime_visual_limit.png" title="Figure 6: Visual limits for amajLime."></a></div> | ||
| + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/1/18/Exeter_aeBlue_visual_limit.png"><img src="https://static.igem.org/mediawiki/2015/1/18/Exeter_aeBlue_visual_limit.png" title="Figure 6: Visual limits for aeBlue."></a></div> | ||
| + | <div class="slide"><a href="https://static.igem.org/mediawiki/2015/2/23/Exeter_eforRed_visual_limit.png"><img src="https://static.igem.org/mediawiki/2015/2/23/Exeter_eforRed_visual_limit.png" title="Figure 6: Visual limits for eforRed."></a></div> | ||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | <p> | ||
| + | Figure 6 shows qualitative data for the colour intensities of the chromoproteins at 50 μg, 10 μg, 5 μg, and 1 μg in 50 μl volumes. | ||
| + | </p> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | </br> | ||
| + | |||
| + | |||
| + | |||
| + | </div> | ||
<!-- This is where my buttons are --> | <!-- This is where my buttons are --> | ||
Latest revision as of 16:55, 1 October 2015
Results
Toehold experiments
In silico testing:
To see an explanation of in silico testing, please visit the modelling page. Download raw data files for Zeus NUPACK testing here. Visit the registry page for more information.Experimental validation of GreenFET1J (BBa_K1586000):
As was explained on the experiments page, GreenJ's (BBa_K1586000) function was characterised and validated by measuring fluorescence intensity of GFP in response to increasing amounts of trigger RNA. Shown in figure 1 is the raw data which was collected from this experiment. In order to analyse this data, the log10 of the amounts of TrigGreen (in nanograms) were calculated. So that the log10 value for 0ng could be calculated, 1x10-6ng was used instead, and all values were increased by six so that 0ng shows as 0 on the graph. These data were then plotted on a graph and a linear trend-line fitted (figure 2).

Further characterisation:
The following parts were further characterised:
Absorbance spectra:
Shown in figure 4 are the absorbance spectra for aeBlue, eforRed, and amajLime between 400nm and 850nm. The peak maxima for these are 595nm, 580nm, and 595nm respectively.
Standard curve - concentration vs. OD:
Figure 5 shows standard curves of concentration in mg ml-1 vs. OD at peak maxima for each of the three chromoproteins further characterised.
Visual limits:
Figure 6 shows qualitative data for the colour intensities of the chromoproteins at 50 μg, 10 μg, 5 μg, and 1 μg in 50 μl volumes.