Team:HKUST-Rice/Application
Application
After designing NPK biosensors and talking with farmers about their needs, we brainstormed potential applications for our system of genetic circuits.
At its inception, the goal of this project was to design a method of determining macronutrient concentrations that was cheap, simple, and easy to use for farmers around the world. However, when we did research on what products were currently available to sense soil macronutrient concentrations, we concluded that many satisfactory, low-cost chemical assays have already been developed. For example, there is a Ferry-Morse Soil Test Kit available for $17 at The Home Depot and schools like Texas A&M offer lab soil tests for $10 per sample.[1,2]
We decided to explore where the current NPK tests could be improved, so we listed out a few of the most essential criteria for a sensor deployment and design. A good sensor for this application is accurate, easy to use, low cost, gives a quick readout of the results, and is composed of relatively stable components.
We then identified two common chemical assays, a colorimetric at-home-kit and laboratory tests, and compared them in each of our design criteria categories. Our analysis showed that, while many of these assays provide cheap and rapid measurement of NPK levels, they do so at the cost of accuracy. We designed the biosensors to be useful registry parts suitable for many future applications and did not specifically design them to improve accuracy. However, we would still like to test if the biological, rather than purely chemical, aspect of our engineered biosensors enables them to get a more accurate reading of what nutrients are readily available from the perspective of a growing plant.
Considering the accessibility of chemical NPK assays and public opinion toward biosensors, we have begun developing two separate applications of the NPK sensors: a paper-based lysate and a controlled biofertilizer system.
Paper-based Lysate
We want to deploy our sensors in a way that would alleviate biosafety concerns and continue to be quick and low-cost so that it continues to be competitive with current products. With these criteria in mind, we decided to make a paper-based testing assay.
We aim to create a freeze-dried cell-free system on paper, based on Dr. Collins’ research, that will be easy to use (Collins paper). Using the liquid tx-tl kit ordered from promega and the associated protocol, we lysed E.Coli expressing mCherry (Protocol). We measured a negative control, positive control, and our sample on a fluorescence plate reader and saw that EXPLAIN FIGURE HERE FIGURE


Moving forward, we would like to move this liquid system to a paper-based system. That way this system can be easily used by farmers. The idea is that after soil is diluted in water, the system can be rehydrated using that water. Then, different colorimetric outputs will be detected by the system depending on the different NPK levels in the soil sample.
Controlled Biofertilizer System
Looking forward, we envisioned an application that would completely remove the need for farmers to constantly test their soil and use fertilizers. Our second application is a system in which a modified soil-bacterium can sense the concentration of a macronutrient in its environment, and then make a decision to add or remove that nutrient from the soil as needed.
Biofertilizer has been defined as the use of microorganisms to increase the availability and uptake of mineral nutrients for plants (Vessey). Depending on the crop, native soil species of nitrogen-fixing, denitrifying, phosphate-solubilizing, and potassium-solubilizing bacteria have been identified (sources). By hooking up our individual nutrient sensors to the nitrate producing machinery of each of these bacterial species, you could theoretically control the increase or decrease that nutrient’s concentration and availability in the soil, depending on the output of the sensor.
We quickly realized that in order to deploy our KPN sensors across multiple strains of soil bacteria, we would need to build the genetic circuitry on broad-host range plasmids. Before putting our sensors into soil bacteria, we decided to show proof-of-concept by using a conjugation plasmid with an RP4 transfer system, green fluorescent protein (GFP), and a chloramphenicol selectable marker. The GFP serves as a visual output.
We then began doing proof-of-concept experiments to transform our broad-host range plasmid containing GFP into two strains of gram-negative soil bacteria: Rhizobia, a nitrogen-fixing microbe, was conjugated to E. coli S17, which along with the conjugation plasmid, provided all the genes necessary to transfer the GFP containing plasmid into the soil bacteria. Additionally, Azotobacter vinelandii, another nitrogen-fixing bacteria, was transformed by electroporation using the conjugation plasmid. Currently, we see that the conjugation of Rhizobia with the E. coli worked since colonies grew on the agarose plates with streptomycin and chloramphenicol antibiotics. The streptomycin ensured that only the Rhizobia grew on the plates since the E. coli were not resistant to streptomycin, and that only Rhizobia with the plasmids grew. In the Azotobacter vinelandii , we have shown that the transformation was successful since colonies grew on plates with the chloramphenicol antibiotic. Only bacteria that would have taken up the plasmid would be resistant. We hope to confirm our findings further by amplifying the nitrogenase gene, only present in Rhizobia and Azotobacter vinelandii. Alternative studies utilizing the plate reader will analyze the fluorescence of the GFP.


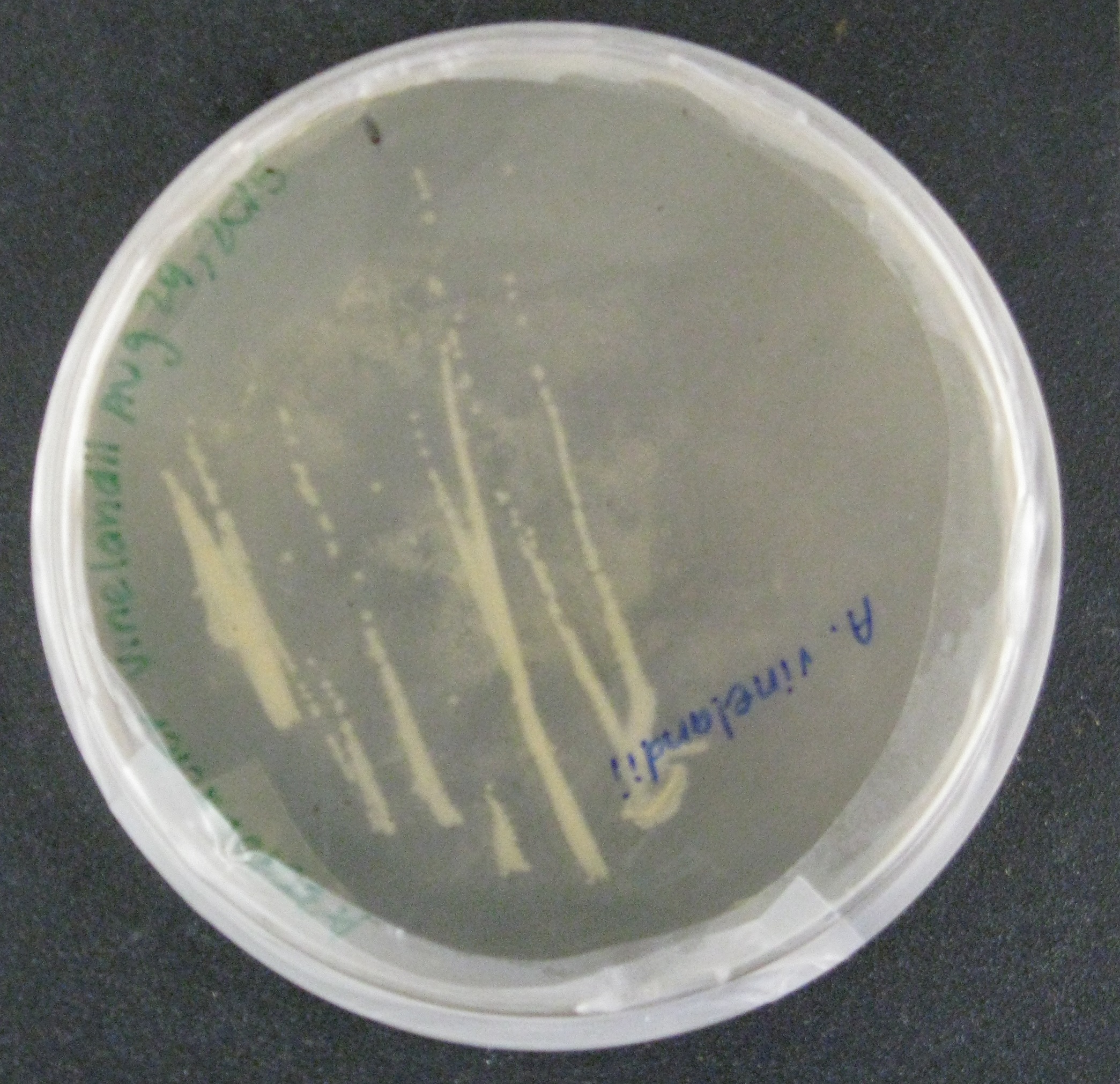
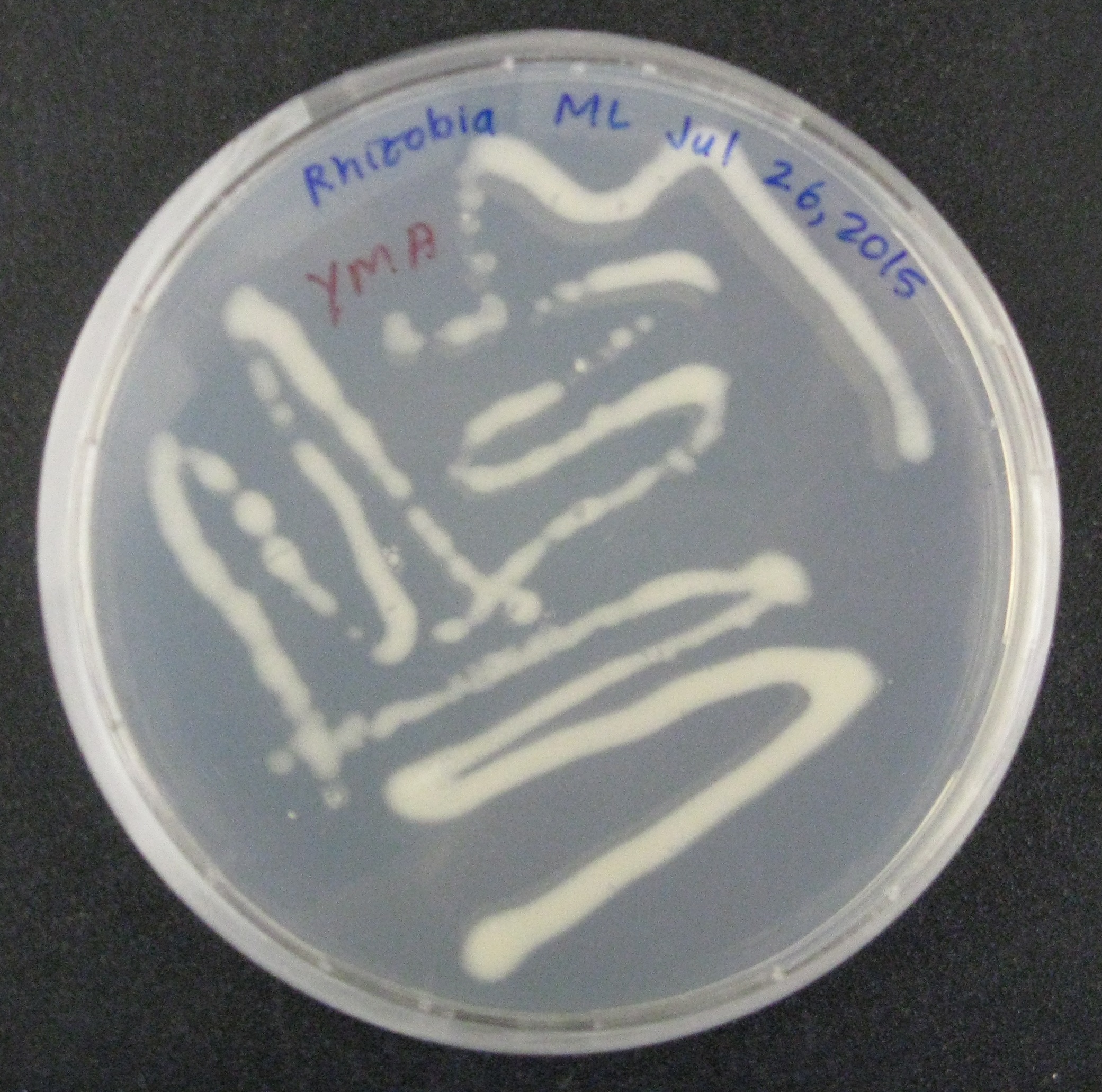
While this application of our NPK sensors is still very much in the initial stages of development, we are very excited by the idea of nutrient-controlled biofertilizer bacteria. This project fully utilizes the potential biocomputing power that can be introduced into bacteria and we hope to continue developing it future years. We envision a day when we resolve biosafety issues and farmers will no longer have to test their soil, determine nutrient deficiencies, and correct them with fertilizers. Instead, our genetically modified soil-bacteria living with the crops will continuously test and replenish or destroy nitrogen, phosphate, and potassium, depending on the specific requirements of the crop. This system would not only make farming easier, by eliminating the need to add fertilizer, it would prevent runoff and have a major positive environmental impact.
References:
1. Ferry Morse 40 Test Soil Test Kit. (n.d.). Retrieved September 8, 2015.
2. Soil Sample Information Form. (n.d.). Retrieved September 8, 2015.
3. Collins, J. (2014). Paper-Based Synthetic Gene Networks. Cell, 159, 940-954.
4. Vessey, J. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil, 571-586.
5. Ngoc Diep, C., & Ngoc Hieu, T. (2013). Phosphate and potassium solubilizing bacteria from weathered materials of denatured rock mountain, Ha Tien, Kiên Giang province, Vietnam. American Journal of Life Sciences, 3(1), 88-92.