Difference between revisions of "Team:TJU/Results"
| Line 184: | Line 184: | ||
<a name="Co-culture MFC" id="Co-culture MFC"></a><h3> 3 Co-culture MFC -- <span style="font-style: normal; color:#ddbf73;">Labor Division</span> </h3> <hr></br> | <a name="Co-culture MFC" id="Co-culture MFC"></a><h3> 3 Co-culture MFC -- <span style="font-style: normal; color:#ddbf73;">Labor Division</span> </h3> <hr></br> | ||
| + | |||
| + | <div class="kuang" style="width:770px"> | ||
| + | </br><a href="https://static.igem.org/mediawiki/2015/d/d7/Result_5.png" target="_blank" ><img src= "https://static.igem.org/mediawiki/2015/d/d7/Result_5.png" width="750" alt=""/></a> | ||
| + | <div id="Enlarge"> | ||
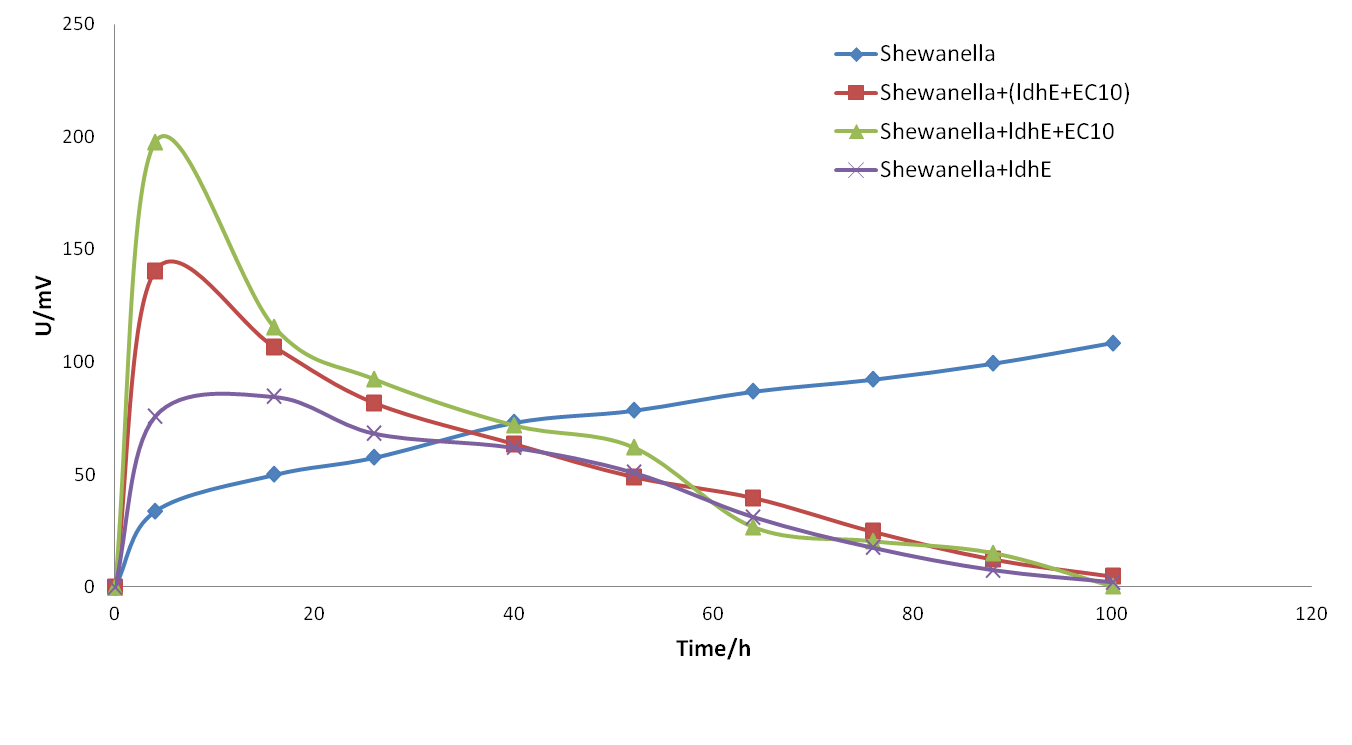
| + | <p> <b>Figure 4.</b> <span style="font-size: 14px"> The electrical output of MFCs of single strain <span style="font-style: italic">Shewanella oneidensis</span> MR-1 with different substrates.</span> | ||
| + | <a href="https://static.igem.org/mediawiki/2015/d/d7/Result_5.png" target="_blank"><img src=" https://static.igem.org/mediawiki/2013/9/90/Enlarge.jpg " width="20" height="20" align="right" alt="" /></a></p></div></div></br> | ||
| + | |||
| + | <p>From the diagram above, we demonstrate that:</p> | ||
| + | <p> 1. <span style="font-style: italic">Shewanella</span> cannot use glucose as a mere carbon substrate to generate power and so the potential keeps no more than 50 mV. </p> | ||
| + | <p> 2. <span style="font-style: italic">Shewanella</span> can produce the maximal potential up to 150mV with supplement of sodium lactate. </p> | ||
| + | <p> 3. <span style="font-style: italic">Shewanella</span> can produce even higher potential when we take sodium lactate as the carbon source and add little riboflavin into the system.</p></br> | ||
| + | <p>Conclusion: According to our experiment, our system is feasible in principle. Lactate can serve as the entry point in material flow and riboflavin as the major factor in energy and information flow, which lays a foundation in the later experiment design. We can use fermentation bacteria to provide carbon sources and electron shuttles, which subsequently, will increase the power generation capability in the co-culture system.</p></br> | ||
| + | |||
| + | <div class="kuang" style="width:770px"> | ||
| + | </br><a href="https://static.igem.org/mediawiki/2015/f/f9/Result_6.png" target="_blank" ><img src= "https://static.igem.org/mediawiki/2015/f/f9/Result_6.png" width="750" alt=""/></a> | ||
| + | <div id="Enlarge"> | ||
| + | <p> <b>Figure 5.</b> <span style="font-size: 14px"> The electrical output of co-culture MFCs with preliminary labor division</span> | ||
| + | <a href="https://static.igem.org/mediawiki/2015/f/f9/Result_6.png" target="_blank"><img src=" https://static.igem.org/mediawiki/2013/9/90/Enlarge.jpg " width="20" height="20" align="right" alt="" /></a></p></div></div></br> | ||
| + | |||
| + | <p>Description: | ||
| + | The results show that dividing material, energy and information flow into separated fermentation bacteria is much better than combining the two tasks into one kind of bacteria. We suppose that the production of riboflavins and lactate cannot be consistent in single strain because lactate is the primary metabolites and riboflavin is the secondary.</p> | ||
| + | |||
| + | <p>However, the electricity output cannot sustain for long and decrease promptly (as shown in figure 5).Based on the measurement, samples from anode reaches an excessively low pH (lower than 6.2) which might not meet the survival requirement of <span style="font-style: italic">Shewanella</span>.</p> | ||
| + | |||
| + | <p>To optimize the system, we try to control the rate of lactate production and adjust the proportion of fermentation bacteria.</p></br> | ||
Figure 12 13 | Figure 12 13 | ||
Revision as of 09:08, 18 September 2015

Results
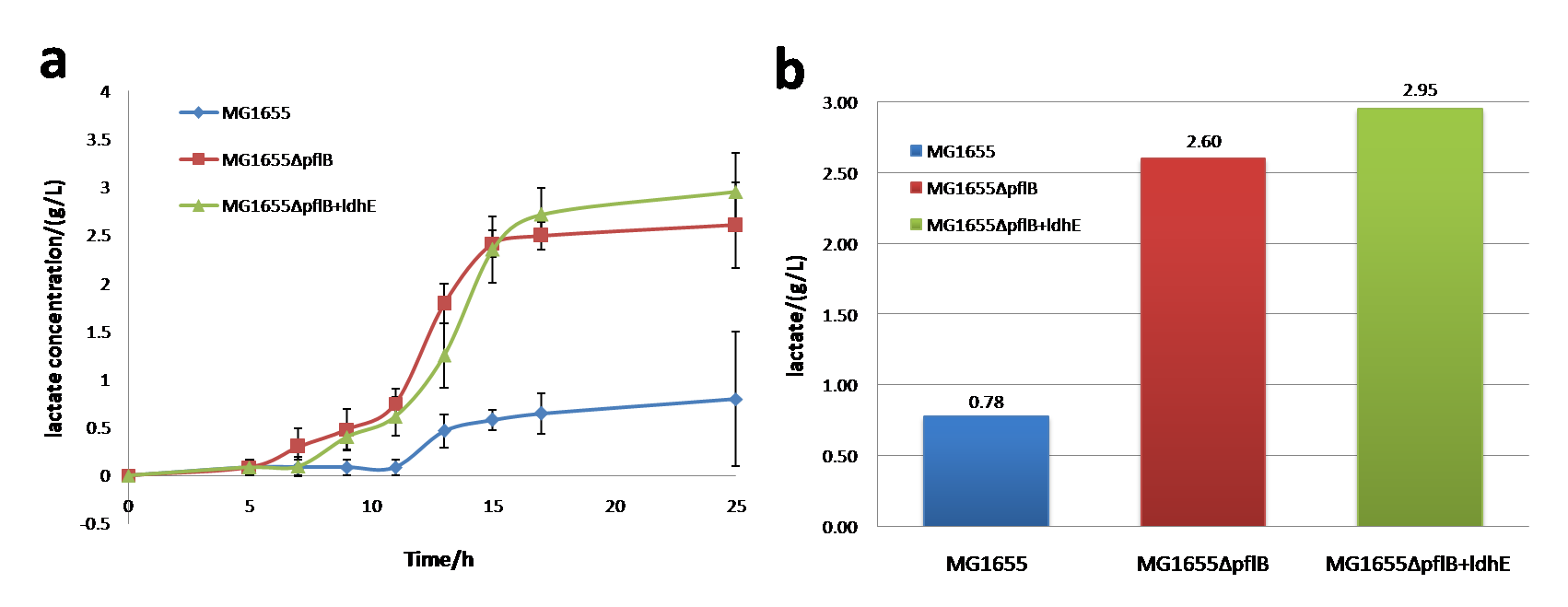
1 Lactate producing system
In order to verify our ldhE part and pflB knockout strategy have works well, we get following results.
Shown as figure 1a, under anaerobic conditions, wild-type MG1655 has the same growth rate as MG1655ΔpflB, but MG1655ΔpflB + ldhE keeps relatively lower growth rate than the two strains mentioned before. Shown as figure 1b, the anaerobic glucose consumption rate keeps the nearly same among MG1655, MG1655ΔpflB and MG1655ΔpflB + ldhE.
In anaerobic environment, lactate production of MG1655, MG1655ΔpflB and MG1655ΔpflB+ldhE has large differences between each other. Notably, knocking out of pflB together with ldhE insertion will increase lactate generation up to ~3 g/L and the amount produced by MG1655ΔpflB is smaller than MG1655ΔpflB+ldhE.
So, knocking out of pflB can dramatically improve the lactate production. Besides, ldhE also plays an important role in lactate increase. Engineered lactate producing strain (MG1655ΔpflB+ldhE) has a greater utilization of carbon sources and a higher yield of lactate with M9 medium under the same concentrations of glucose.
2 Flavins producing system
To prove the EC10 and EC10* work as expected, we conduct several experiments and the results are as follows.
As shown in figure 3, E. coli BL21 with EC10 reaches a final yield of 17 mg/L in tube cultivation. E. coli BL21Δpgi + EC10 reaches 33 mg/L. E. coli BL21 + EC10* reaches 90 mg/L.
3 Co-culture MFC -- Labor Division
From the diagram above, we demonstrate that:
1. Shewanella cannot use glucose as a mere carbon substrate to generate power and so the potential keeps no more than 50 mV.
2. Shewanella can produce the maximal potential up to 150mV with supplement of sodium lactate.
3. Shewanella can produce even higher potential when we take sodium lactate as the carbon source and add little riboflavin into the system.
Conclusion: According to our experiment, our system is feasible in principle. Lactate can serve as the entry point in material flow and riboflavin as the major factor in energy and information flow, which lays a foundation in the later experiment design. We can use fermentation bacteria to provide carbon sources and electron shuttles, which subsequently, will increase the power generation capability in the co-culture system.
Description: The results show that dividing material, energy and information flow into separated fermentation bacteria is much better than combining the two tasks into one kind of bacteria. We suppose that the production of riboflavins and lactate cannot be consistent in single strain because lactate is the primary metabolites and riboflavin is the secondary.
However, the electricity output cannot sustain for long and decrease promptly (as shown in figure 5).Based on the measurement, samples from anode reaches an excessively low pH (lower than 6.2) which might not meet the survival requirement of Shewanella.
To optimize the system, we try to control the rate of lactate production and adjust the proportion of fermentation bacteria.
Figure 12 13The relations between current density and voltage is referred as polarization curve while the power curve can represent the relations between output power density and current density. We can learn from the fugure ***, the highest power density of (1) reaches 10 mW/m2 and (2) reaches 17 mW/m2.
Figure 14 15It is obvious that the power output in three-strain MFC systems with a more complete labor division are far greater than single-strain and two-strian MFCs.
Figure 16It has been shown in power curve that Shewanella + ΔplfB ldhE + B. Subtilis has a significantly higher output than Shewanella + ΔplfB ldhE + Rf02S, which indicates that three-species system can generate higher electricity and have a better MFC performance, in return, have a promising application.
E-mail: ggjyliuyue@gmail.com |Address: Building No.20, No.92 Weijin road, Tianjin University, China | Zip-cod: 300072
Copyright 2015@TJU iGEM Team