Team:TJU/Collaborations


1 High Schools
----Mentoring new high school teams
This year, we publicize an assistant Handbook in Chinese version striving to help general schools get familiar with the iGEM competition and encourage them to take part in. Particularly, our handbook focuses on the basic information like long-standing history and medal requirements, as well as the outstanding works done by others. Beside, this book is practical for anyone who wants to know more about iGEM with easy-to-approach pictures and summaries which then gives readers a clear image about this competition. Our Handbook has helped five high schools including SKLBC-GDSYZX, SKLBC-China, Shiyan_SY_China, H4Z-Hangzhou and WLSA_Shanghai as well as several universities. Notably, SKLBC-GDSYZX started their first journey in iGEM this year within our collaboration. They benefit a lot especially from team setup guidance since it is their first time to organize an iGEM team. Questions like how to gather the talents and how to figure out their topic seem important for them. Although other teams are not the first time to participate in, they also learn a lot from our experience. They highly valued our work exhibition sector and paid more attention studying deeply. We believe that our Handbook can prompt their success and we are intended to help even more schools not just in China but all over the world.
Check the wiki of SKLBC-GDSYZX to view our collaboration:
Here are some photos and feedbacks from different high schools:
2 LZU-China
----Characterizing their new parts
In 2015’s iGEM competition, we collaborate with LZU-China from Lanzhou University. In August 2015, LZU-China visited Tianjin University and we held a tiny meet-up. We presented the project station and provided many valuable advises for each other.
In the meeting, we knew that LZU-China constructed many sensors to sense the pollutes in liquids. The sensors are used in MFCs (microbial fuel cell). When pollutes appear, the sensors can produce riboflavin to improve the voltage. We are very pleased to find both of us work on the MFC system so that we can share many experience and problems. In this year’s project. LZU-China try to develop a system based on MFC to sense the pollutes. They constructed a series of heavy metal sensors used in MFCs (microbial fuel cell). When pollutes appear, the sensors can produce riboflavin to improve the voltage. Through our research, we find that F- is also a remarkable pollute which have a potential risk to human beings so we advised them to construct a F- sensor and the sensor can be constructed based on BBa_K911003. Through our advice, LZU-China then constructed BBa_K1755024, the F- sensor.
F- sensing part
To prove their part BBa_K1755024 can work normally, we tested their part in our own lab under our MFC system which had similar culture environment. The BL21 with K1755024 was cultured added with NaF and the production of riboflavin was measured.
Firstly, we measured the riboflavin concentration produced by BBa_K1755024. 3ml bacteria solution was taken every 12 hours(0 h, 12 h, 24 h, 36 h, 48 h), their absorbance (OD values ) were measured in the 600 nm wavelength. The absorbance of supernatant (OD values ) were measured in the 444 nm wavelength, which suggests the concentration of riboflavin.
We can see from the figure that though the background expression is relatively high, the concentration of riboflavin shows a good linear relationship with that of NaF when NaF ranges from 0 to 40 μm. However, 80 μm condition, through our speculation, may inhibit the expression of riboflavin.
Cu2+ sensing part
Apart from the F- part, we also helped them characterize the Cu2+ sensing part in our MFC device.
As for BBa_K1755301, the voltage in MFC was documented. The MFC‘s anode was added with Cu2+ from the very begging (0 min), and their voltage (with BL21-K1755301) was also documented.
The results above show a significant difference of the voltage output at around 120 min. After 240 min, the voltage output became closer. So, the Cu2+ sensing part proves to be functional.
We can see that the E.coli, modified by the part they constructed can sense the concentration of Cu ions in different labs normally. The data shows typically consistent.This also indicates the biological component of LZU-China’s “Micro saHolmes” measurement system has nice accessibility and portability.(Table-1)
References
[1]Oliveira, V. B., Simões, M., Melo, L. F., and Pinto, A. M. F. R. (2013) Biochemical Engineering Journal 73, 53-64 [2]Rabaey, K., and Verstraete, W. Trends in Biotechnology 23, 291-298 [3]Zhang, T., Cui, C., Chen, S., Ai, X., Yang, H., Shen, P., and Peng, Z. (2006) Chemical Communications [4]Jenny L. Baker et al., Widespread Genetic Switches and Toxicity Resistance Proteins for Fluoride, Science (2012) vol. 335 pp. 233 - 235.
3 BIT-China 
----Modeling their system
BIT-CHINA this year designs an intelligent pH-Controller inside microorganism tailored to earn the ability for pH regulation in industrial fermentation. During our conversation, they have heard that some part of our model is related to pH induction and regulation, which is closely connected with their efforts. Meanwhile, they have also experienced a lot of difficulties when constructing their model. As a collaboration to improve the models on both side, we respond to their request and help them as much as possible.
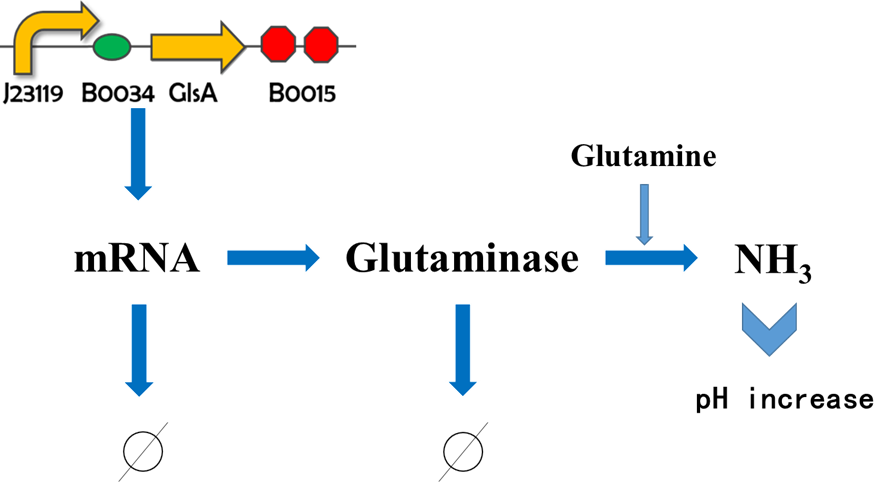
The basic principles of pH tolerance
1. Under acid conditions, the acid-resisting gene glsA can produce glutaminase which catalyzes Gln to generate NH3 as well as Glu. Subsequently, NH3 is able to neutralize H+ inside the cell resulted in pH ascending. 2. When intracellular pH goes up, the alkali-resisting genes NhaA and NhaB can generate Na+/H+ pump which transfers Na+ to the outside in exchange for H+ from the outside. Thus, H+ is able to neutralize OH- inside the cell leading to pH drop. Next, we will demonstrate our construction via taking the pH regulation module as an example.
We establish the following equations based on the gene circle above:
As a step forward, we established the following ordinary differential equations on the basis of Law of Conservation of Mass and Michaelis-Menten equation:
Supposing that the original pH in the external environment is 4, we can get the picture of intracellular H+ concentration change (as shown in picture 1).
the figure indicates that the intracellular acid concentration tends to be stable, which clarifies that the acid controller can make microorganism more tolerant in the low pH conditions. Besides, the principles and equations used in alkali controller modeling is similar to those used in acid controller.
E-mail: ggjyliuyue@gmail.com |Address: Building No.20, No.92 Weijin road, Tianjin University, China | Zip-cod: 300072
Copyright 2015@TJU iGEM Team